Cell cycle progression and proliferation despite 4BP-1 dephosphorylation
- PMID: 10454551
- PMCID: PMC84502
- DOI: 10.1128/MCB.19.9.6041
Cell cycle progression and proliferation despite 4BP-1 dephosphorylation
Abstract
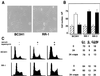
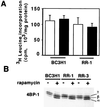
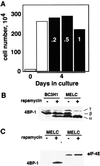
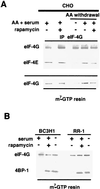
Proliferation and cell cycle progression in response to growth factors require de novo protein synthesis. It has been proposed that binding of the eukaryotic translation initiation factor 4E (eIF-4E) to the inhibitory protein 4BP-1 blocks translation by preventing access of eIF-4G to the 5' cap of the mRNA. The signal for translation initiation is thought to involve phosphorylation of 4BP-1, which causes it to dissociate from eIF-4E and allows eIF-4G to localize to the 5' cap. It has been suggested that the ability of the macrolide antibiotic rapamycin to inhibit 4BP-1 phosphorylation is responsible for the potent antiproliferative property of this drug. We now show that rapamycin-resistant cells exhibited normal proliferation despite dephosphorylation of 4BP-1 that allows it to bind to eIF-4E. Moreover, despite rapamycin-induced dephosphorylation of 4BP-1, eIF-4E-eIF-4G complexes (eIF-4F) were still detected. In contrast, amino acid withdrawal, which caused a similar degree of 4BP-1 dephosphorylation, resulted in dissociation of the eIF-4E-eIF-4G complex. Thus, 4BP-1 dephosphorylation is not equivalent to eIF-4E inactivation and does not explain the antiproliferative property of rapamycin.
Figures


References
-
- Blackshear P J, Stumpo D J, Carballo E, Lawrence J C. Disruption of the gene encoding the mitogen-regulated translational modulator PHAS-I in mice. J Biol Chem. 1997;272:31510–31514. - PubMed
-
- Blommaart E F, Luiken J J, Blommaart P J, van Woerkom G M, Meijer A J. Phosphorylation of ribosomal protein S6 is inhibitory for autophagy in isolated rat hepatocytes. J Biol Chem. 1995;270:2320–2326. - PubMed
-
- Brown E, Albers T, Shin T, Ichikawa K, Keith C, Lane W, Schreiber S. A mammalian protein targeted by G1-arresting rapamycin complex. Nature (London) 1994;369:756–758. - PubMed
-
- Brown E J, Beal P A, Keith C T, Chen J, Shin T B, Schreiber S L. Control of p70 S6 kinase by kinase activity of FRAP in vivo. Nature (London) 1995;377:441–446. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
