The androgen receptor amino-terminal domain plays a key role in p160 coactivator-stimulated gene transcription
- PMID: 10454556
- PMCID: PMC84524
- DOI: 10.1128/MCB.19.9.6085
The androgen receptor amino-terminal domain plays a key role in p160 coactivator-stimulated gene transcription
Abstract
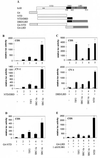
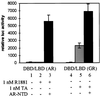
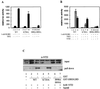
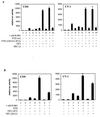
Steroid receptors are conditional transcription factors that, upon binding to their response elements, regulate the expression of target genes via direct protein interactions with transcriptional coactivators. We have analyzed the functional interactions between the androgen receptor (AR) and 160-kDa nuclear receptor coactivators. Upon overexpression in mammalian cells, these coactivators enhance the transcriptional activity of both the amino-terminal domain (NTD) and the ligand-binding domain (LBD) of the AR. The coactivator activity for the LBD is strictly ligand-controlled and depends on the nature of the DNA-binding domain to which it is fused. We demonstrate that the NTD physically interacts with coactivators and with the LBD and that this interaction, like the functional interaction between the LBD and p160 coactivators, relies on the activation function 2 (AF2) core domain. The mutation of a highly conserved lysine residue in the predicted helix 3 of the LBD (K720A), however, blunts the functional interaction with coactivators but not with the NTD. Moreover, this mutation does not affect the transcriptional activity of the full-size AR. A mutation in the NTD of activation function AF1a (I182A/L183A), which dramatically impairs the activity of the AR, has no effect on the intrinsic transcriptional activity of the NTD but interferes with the cooperation between the NTD and the LBD. Finally, p160 proteins in which the three LXXLL motifs are mutated retain most of their coactivator activity for the full-size AR, although they are no longer functional for the isolated LBD. Together, these data suggest that in the native AR the efficient recruitment of coactivators requires a functional association of the NTD with the LBD and that the binding of coactivators occurs primarily through the NTD.
Figures
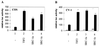
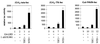

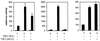

References
-
- Alen P, Claessens F, Schoenmakers E, Swinnen J V, Verhoeven G, Rombauts W, Peeters B. Interaction of the putative androgen receptor-specific coactivator ARA70/ELE1α with multiple steroid receptors and identification of an internally deleted ELE1β isoform. Mol Endocrinol. 1999;13:117–128. - PubMed
-
- Anzick S L, Kononen J, Walker R L, Azorsa D O, Tanner M M, Guan X Y, Sauter G, Kallioniemi O P, Trent J M, Meltzer P S. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science. 1997;277:965–968. - PubMed
-
- Bannister A J, Kouzarides T. The CBP co-activator is a histone acetyltransferase. Nature. 1996;384:641–643. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
