MCM proteins are associated with RNA polymerase II holoenzyme
- PMID: 10454562
- PMCID: PMC84545
- DOI: 10.1128/MCB.19.9.6154
MCM proteins are associated with RNA polymerase II holoenzyme
Abstract
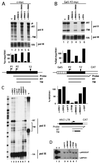
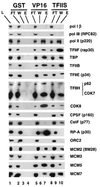
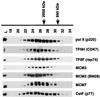
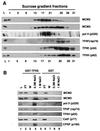
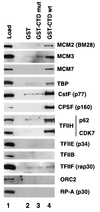
MCMs are a family of proteins related to ATP-dependent helicases that bind to origin recognition complexes and are required for initiation of DNA replication. We report that antibodies against MCM2(BM28) specifically inhibited transcription by RNA polymerase II (Pol II) in microinjected Xenopus oocytes. Consistent with this observation, MCM2 and other MCMs copurified with Pol II and general transcription factors (GTFs) in high-molecular-weight holoenzyme complexes isolated from Xenopus oocytes and HeLa cells. Pol II and GTFs also copurified with MCMs isolated by anti-MCM3 immunoaffinity chromatography. MCMs were specifically displaced from the holoenzyme complex by antibody against the C-terminal domain (CTD) of Pol II. In addition, MCMs bound to a CTD affinity column, suggesting that their association with holoenzyme depends in part on this domain of Pol II. These results suggest a new function for MCM proteins as components of the Pol II transcriptional apparatus.
Figures



Similar articles
-
Distinct parts of minichromosome maintenance protein 2 associate with histone H3/H4 and RNA polymerase II holoenzyme.Eur J Biochem. 2002 Nov;269(21):5192-202. doi: 10.1046/j.1432-1033.2002.03224.x. Eur J Biochem. 2002. PMID: 12392551
-
Splicing and transcription-associated proteins PSF and p54nrb/nonO bind to the RNA polymerase II CTD.RNA. 2002 Sep;8(9):1102-11. doi: 10.1017/s1355838202025037. RNA. 2002. PMID: 12358429 Free PMC article.
-
The minichromosome maintenance proteins 2-7 (MCM2-7) are necessary for RNA polymerase II (Pol II)-mediated transcription.J Biol Chem. 2009 May 15;284(20):13466-13472. doi: 10.1074/jbc.M809471200. Epub 2009 Mar 23. J Biol Chem. 2009. PMID: 19318354 Free PMC article.
-
Histone acetyltransferase and protein kinase activities copurify with a putative Xenopus RNA polymerase I holoenzyme self-sufficient for promoter-dependent transcription.Mol Cell Biol. 1999 Jan;19(1):796-806. doi: 10.1128/MCB.19.1.796. Mol Cell Biol. 1999. PMID: 9858602 Free PMC article.
-
Binding of human minichromosome maintenance proteins with histone H3.J Biol Chem. 1996 Sep 27;271(39):24115-22. doi: 10.1074/jbc.271.39.24115. J Biol Chem. 1996. PMID: 8798650
Cited by
-
Inhibition of HTLV-1 transcription by cyclin dependent kinase inhibitors.Mol Cell Biochem. 2002 Aug;237(1-2):137-53. doi: 10.1023/a:1016555821581. Mol Cell Biochem. 2002. PMID: 12236581
-
Identification of two residues in MCM5 critical for the assembly of MCM complexes and Stat1-mediated transcription activation in response to IFN-gamma.Proc Natl Acad Sci U S A. 2001 Mar 13;98(6):3034-9. doi: 10.1073/pnas.061487598. Epub 2001 Mar 6. Proc Natl Acad Sci U S A. 2001. PMID: 11248027 Free PMC article.
-
Transcription shapes DNA replication initiation to preserve genome integrity.Genome Biol. 2021 Jun 9;22(1):176. doi: 10.1186/s13059-021-02390-3. Genome Biol. 2021. PMID: 34108027 Free PMC article.
-
MCM Paradox: Abundance of Eukaryotic Replicative Helicases and Genomic Integrity.Mol Biol Int. 2014;2014:574850. doi: 10.1155/2014/574850. Epub 2014 Oct 19. Mol Biol Int. 2014. PMID: 25386362 Free PMC article. Review.
-
Preventing re-replication of chromosomal DNA.Nat Rev Mol Cell Biol. 2005 Jun;6(6):476-86. doi: 10.1038/nrm1663. Nat Rev Mol Cell Biol. 2005. PMID: 15928711 Free PMC article. Review.
References
-
- Anderson S F, Schlegel B P, Nakajima T, Wolpin E S, Parvin J D. BRCA1 protein is linked to the RNA polymerase II holoenzyme complex via RNA helicase A. Nat Genet. 1998;19:254–256. - PubMed
-
- Aparicio O, Weinstein O, Bell S. Components and dynamics of DNA replication complexes in S. cerevisiae: redistribution of MCM proteins and cdc45 during S phase. Cell. 1997;91:59–69. - PubMed
-
- Bentley D L, Brown W L, Groudine M. Accurate, TATA box-dependent polymerase III transcription from promoters of the c-myc gene in injected Xenopus oocytes. Genes Dev. 1989;3:1179–1189. - PubMed
-
- Blow J J, Laskey R A. A role for the nuclear envelope in controlling DNA replication within the cell cycle. Nature. 1988;332:546–548. - PubMed
-
- Burkhart R, Schulte D, Hu D, Musahl C, Goehring F, Knippers R. Interactions of human nuclear proteins P1Mcm3 and P1Cdc46. Eur J Biochem. 1995;228:431–438. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
