Ectopic expression of Cdc25A accelerates the G(1)/S transition and leads to premature activation of cyclin E- and cyclin A-dependent kinases
- PMID: 10454565
- PMCID: PMC84557
- DOI: 10.1128/MCB.19.9.6183
Ectopic expression of Cdc25A accelerates the G(1)/S transition and leads to premature activation of cyclin E- and cyclin A-dependent kinases
Abstract
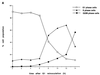
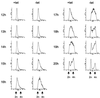
Human Cdc25 phosphatases play important roles in cell cycle regulation by removing inhibitory phosphates from tyrosine and threonine residues of cyclin-dependent kinases. Three human Cdc25 isoforms, A, B, and C, have been discovered. Cdc25B and Cdc25C play crucial roles at the G(2)/M transition. In the present study, we have investigated the function of human Cdc25A phosphatase. Cell lines that express human Cdc25A in an inducible manner have been generated. Ectopic expression of Cdc25A accelerates the G(1)/S-phase transition, indicating that Cdc25A controls an event(s) that is rate limiting for entry into S phase. Furthermore, we carried out a detailed analysis of the expression and activation of human Cdc25A. Activation of endogenous Cdc25A occurs during late G(1) phase and increases in S and G(2) phases. We further demonstrate that Cdc25A is activated at the same time as cyclin E- and cyclin A-dependent kinases. In vitro, Cdc25A dephosphorylates and activates the cyclin-Cdk complexes that are active during G(1). Overexpression of Cdc25A in the inducible system, however, leads to a premature activation of both cyclin E-Cdk2 and cyclin A-Cdk2 complexes, while no effect of cyclin D-dependent kinases is observed. Furthermore, Cdc25A overexpression induces a tyrosine dephosphorylation of Cdk2. These results suggest that Cdc25A is an important regulator of the G(1)/S-phase transition and that cyclin E- and cyclin A-dependent kinases act as direct targets.
Figures










References
-
- Draetta G, Beach D. Activation of cdc2 protein kinase during mitosis in human cells: cell cycle-dependent phosphorylation and subunit rearrangement. Cell. 1988;54:17–26. - PubMed
-
- Fauman E, Cogswell J, Lovejoy B, Rocque W, Holmes W, Montana V, Piwnica-Worms H, Rink M, Saper M. Crystal structure of the catalytic domain of the human cell cycle control phosphatase, Cdc25A. Cell. 1998;93:617–625. - PubMed
-
- Gabrielli B, De Souza C, Tonks I, Clark J, Hayward N, Ellem K. Cytoplasmic accumulation of Cdc25B phosphatase in mitosis triggers centrosomal microtubule nucleation in HeLa cells. J Cell Sci. 1996;109:1081–1093. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
