Pharmacological characterization of extracellular acidification rate responses in human D2(long), D3 and D4.4 receptors expressed in Chinese hamster ovary cells
- PMID: 10455259
- PMCID: PMC1566129
- DOI: 10.1038/sj.bjp.0702657
Pharmacological characterization of extracellular acidification rate responses in human D2(long), D3 and D4.4 receptors expressed in Chinese hamster ovary cells
Abstract
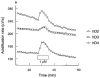
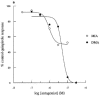
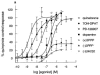
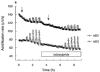
This study characterized pharmacologically the functional responses to agonists at human dopamine D2(long) (hD2), D3 (hD3) and D4.4 (hD4) receptors separately expressed in cloned cells using the cytosensor microphysiometer. Dopaminergic receptor agonists caused increases in extracellular acidification rate in adherent Chinese hamster ovary (CHO) clones expressing hD2, hD3 or hD4 receptors. Acidification rate responses to agonists in other cell lines expressing these receptors were smaller than those in adherent CHO cells. The time courses and maximum increases in acidification rate of the agonist responses in adherent CHO cells were different between the three dopamine receptor clones. Responses were blocked by pretreatment of cells with pertussis toxin or amiloride analogues. Most agonists had full intrinsic activity at each of the dopamine receptor subtypes, as compared to quinpirole, however both enantiomers of UH-232 and (-)3-PPP were partial agonists in this assay system. The functional potency of full agonists at each of the three receptors expressed in CHO cells was either higher than, or similar to, the apparent inhibition constants (Ki) determined in [125I]-iodosulpride competition binding studies. Functional selectivities of the agonists were less than radioligand binding selectivities. The rank orders of agonist potencies and selectivities were similar, but not identical, to the rank orders of radioligand binding affinities and selectivities. The dopamine receptor antagonists, iodosulpride and clozapine, had no effect on basal acidification rates but inhibited acidification responses in CHO cells to quinpirole in an apparently competitive manner. Antagonist potencies closely matched their radioligand binding affinities in these cells.
Figures









References
-
- ASGHARI V., SANYAL S., BUCHWALDT S., PATERSON A., JOVANOVIC V., VAN TOL H. Modulation of intracellular cyclic AMP levels by different human dopamine D4 receptor variants. J. Neurochem. 1995;65:1157–1165. - PubMed
-
- BOWEN W.P., COLDWELL M.C., HICKS F.R., RILEY G.J. Further characterisation of human D2 and D3 receptors-GppNHp shifts are explained by the presence of more than one binding site in each clone. Br. J. Pharmacol. 1993;108:277P.
-
- BOWEN W.P., JERMAN J.C. Nonlinear regression using spreadsheets. Trends Pharmacol. Sci. 1995;16:413–417. - PubMed
-
- BOYFIELD I., WINN F., COLDWELL M.C. Comparison of agonist potencies at human D2 and D3 receptors, expressed in the same cell line, using the Cytosensor microphysiometer. Biochem. Soc. Trans. 1996;24:57S. - PubMed
-
- BURRIS K.D., PACHECO M.A., FILTZ T.M., KUNG M.-P., KUNG H.F., MOLINOFF P.B. Lack of discrimination by agonists for D2 and D3 dopamine receptors. Neuropsychopharmacology. 1995;12:335–345. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

