Involvement of ATP-sensitive potassium channels in a model of a delayed vascular hyporeactivity induced by lipopolysaccharide in rats
- PMID: 10455295
- PMCID: PMC1760645
- DOI: 10.1038/sj.bjp.0702666
Involvement of ATP-sensitive potassium channels in a model of a delayed vascular hyporeactivity induced by lipopolysaccharide in rats
Abstract
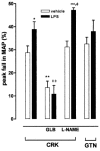
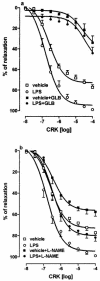
We have investigated the role of ATP-sensitive potassium (K(ATP)) channels in an experimental model of a delayed phase of vascular hyporeactivity induced by lipopolysaccharide (LPS) in rats. After 24 h, from LPS treatment, in anaesthetized rats the bolus injection of phenylephrine (PE) produced an increase in mean arterial pressure (MAP) significantly (P<0.05) reduced in LPS-treated rats compared to the vehicle-treated rats. This reduction was prevented by pre-treatment of rats with glibenclamide (GLB), a selective inhibitor of K(ATP) channels. GLB administration did not affect the MAP in vehicle-treated rats but produced an increase of MAP in rats treated with LPS. Cromakalim (CRK), a selective K(ATP) channel opener, produced a reduction of MAP that was significantly (P<0.05) higher in LPS- than in vehicle-treated rats. In contrast, the hypotension induced by glyceryl trinitrate (GTN) in LPS-treated rats was not distinguishable from that produced in vehicle-treated rats. Experiments in vitro were conducted on aorta rings collected from rats treated with vehicle or LPS 24 h before sacrifice. The concentration-dependent curve to PE was statistically (P<0.005) reduced in aorta rings collected from LPS- compared to vehicle-treated rats. This difference was totally abolished by tetraethylammonium (TEA), a non-selective inhibitor of K+ channels. CRK produced a relaxation of PE precontracted aorta rings higher in rings from LPS- than in vehicle-treated rats. GLB inhibited CRK-induced relaxation in both tissues, abolishing the observed differences. In conclusion, our results indicate an involvement of K(ATP) channels to the hyporesponsiveness of vascular tissue after 24 h from a single injection of LPS in rats. We can presume an increase in the activity of K(ATP) channels on vascular smooth muscle cells but we cannot exclude an increase of K(ATP) channel number probably due to the gene expression activation.
Figures



Similar articles
-
Metamizol acts as an ATP sensitive potassium channel opener to inhibit the contracting response induced by angiotensin II but not to norepinephrine in rat thoracic aorta smooth muscle.Vascul Pharmacol. 2005 Aug;43(2):120-7. doi: 10.1016/j.vph.2005.05.003. Vascul Pharmacol. 2005. PMID: 15958287
-
A xanthine-based KMUP-1 with cyclic GMP enhancing and K(+) channels opening activities in rat aortic smooth muscle.Br J Pharmacol. 2001 Sep;134(2):265-74. doi: 10.1038/sj.bjp.0704231. Br J Pharmacol. 2001. PMID: 11564644 Free PMC article.
-
Abnormal activation of K+ channels underlies relaxation to bacterial lipopolysaccharide in rat aorta.Biochem Biophys Res Commun. 1996 Jul 5;224(1):184-90. doi: 10.1006/bbrc.1996.1005. Biochem Biophys Res Commun. 1996. PMID: 8694810
-
Functional roles of KATP channels in vascular smooth muscle.Clin Exp Pharmacol Physiol. 2002 Apr;29(4):312-6. doi: 10.1046/j.1440-1681.2002.03650.x. Clin Exp Pharmacol Physiol. 2002. PMID: 11985542 Review.
-
Modulation of muscle contractility during fatigue and recovery by ATP sensitive potassium channel.Acta Physiol Scand. 1996 Mar;156(3):203-12. doi: 10.1046/j.1365-201X.1996.210000.x. Acta Physiol Scand. 1996. PMID: 8729680 Review.
Cited by
-
Is There NO Treatment For Severe Sepsis?Libyan J Med. 2008 Mar 1;3(1):34-8. doi: 10.4176/071018. Libyan J Med. 2008. PMID: 21499479 Free PMC article.
-
HMR1402, a potassium ATP channel blocker during hyperdynamic porcine endotoxemia: effects on hepato-splanchnic oxygen exchange and metabolism.Intensive Care Med. 2004 May;30(5):957-64. doi: 10.1007/s00134-004-2258-9. Epub 2004 Mar 26. Intensive Care Med. 2004. PMID: 15045166
-
Dexamethasone improves vascular hyporeactivity induced by LPS in vivo by modulating ATP-sensitive potassium channels activity.Br J Pharmacol. 2003 Sep;140(1):91-6. doi: 10.1038/sj.bjp.0705406. Epub 2003 Aug 4. Br J Pharmacol. 2003. PMID: 12967938 Free PMC article.
-
Curcumin protects against lipopolysaccharide-induced vasoconstriction dysfunction via inhibition of thrombospondin-1 and transforming growth factor-β1.Exp Ther Med. 2015 Feb;9(2):377-383. doi: 10.3892/etm.2014.2105. Epub 2014 Dec 3. Exp Ther Med. 2015. PMID: 25574201 Free PMC article.
-
The pore-forming subunit of the K(ATP) channel is an important molecular target for LPS-induced vascular hyporeactivity in vitro.Br J Pharmacol. 2005 Feb;144(3):367-75. doi: 10.1038/sj.bjp.0706065. Br J Pharmacol. 2005. PMID: 15655519 Free PMC article.
References
-
- BENTLER B.Cachetin/Tumor necrosis factor and lymphotoxin Handbook of Experimental Pharmacology Peptide Growth Factors and Their Receptor II 1990Heidelberg: Springer-Verlag; 39–70.ed. Sporn, M.B. & Roberts, A.B. pp
-
- BIGAUD M., JOULOU-SCHAEFFER G., PARRATT J.R., STOCLET J. Endotoxin-induced impairment of vascular smooth muscle contractions elicited by different mechanisms. Eur. J. Pharmacol. 1990;190:185–192. - PubMed
-
- ETIENNE A., HECQUET C., SOULARD C., TOUVAY F., CLOSTRE F., BRAQUET P. The relative role of PAF-acether and eicosanoids in septic shock. Pharmacol. Res. Commun. 1986;18 Suppl:71–79. - PubMed
-
- GIROIR B.P. Mediators of septic shock: New approaches for interrupting the endogenous inflammatory cascade. Crit. Care Med. 1993;25:780–789. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

