Spinal effect of a neuropeptide FF analogue on hyperalgesia and morphine-induced analgesia in mononeuropathic and diabetic rats
- PMID: 10455296
- PMCID: PMC1760663
- DOI: 10.1038/sj.bjp.0702682
Spinal effect of a neuropeptide FF analogue on hyperalgesia and morphine-induced analgesia in mononeuropathic and diabetic rats
Abstract
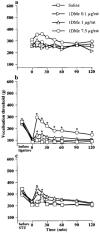
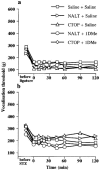
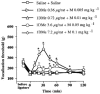
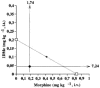
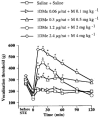
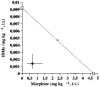
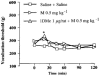
1DMe, a neuropeptide FF (NPFF) analogue, has been shown to produce antinociception and to enhance morphine analgesia in rats after intrathecal administration. To determine whether 1DMe could correct hyperalgesia and restore morphine efficacy in mononeuropathic (MN) and diabetic (D) rats we examined the spinal effect of 1DMe in MN and D rats without and after spinal blockade of mu- and delta-opioid receptors with CTOP and naltrindole, respectively. The influence of 1DMe on morphine-induced antinociception was assessed in the two models using isobolographic analysis. Whereas 1DMe intrathecally injected (0.1, 1, 7.5 microg rat(-1)) was ineffective in normal (N) rats, it suppressed mechanical hyperalgesia (decrease in paw pressure-induced vocalisation thresholds) in both MN and D rats. This effect was completely cancelled by CTOP (10 microg rat(-1)) and naltrindole (1 microg rat(-1)) suggesting that it requires the simultaneous availability of mu- and delta-opioid receptors. The combinations of morphine: 1DMe (80.6:19.4% and 99.8:0.2%, in MN and D rats, respectively) followed by isobolographic analysis, showed a superadditive interaction, relative to the antinociceptive effect of single doses, in D rats only. In N rats, the combination of morphine: 1DMe (0.5 mg kg(-1), i.v.: 1 microg rat(-1), i.t., ineffective doses) resulted in a weak short-lasting antinociceptive effect. These results show a different efficacy of 1DMe according to the pain model used, suggesting that the pro-opioid effects of the NPFF in neuropathic pain are only weak, which should contribute to hyperalgesia and to the impaired efficacy of morphine.
Figures
Similar articles
-
Role of opioid receptors in the spinal antinociceptive effects of neuropeptide FF analogues.Br J Pharmacol. 1996 Feb;117(3):493-501. doi: 10.1111/j.1476-5381.1996.tb15217.x. Br J Pharmacol. 1996. PMID: 8821539 Free PMC article.
-
Evidence for an exclusive antinociceptive effect of nociceptin/orphanin FQ, an endogenous ligand for the ORL1 receptor, in two animal models of neuropathic pain.Pain. 2004 Jul;110(1-2):236-45. doi: 10.1016/j.pain.2004.03.037. Pain. 2004. PMID: 15275773
-
Spinal effect of the cholecystokinin-B receptor antagonist CI-988 on hyperalgesia, allodynia and morphine-induced analgesia in diabetic and mononeuropathic rats.Pain. 2000 Oct;88(1):15-22. doi: 10.1016/S0304-3959(00)00304-3. Pain. 2000. PMID: 11098095
-
Neuropeptide FF, pain and analgesia.Eur J Pharmacol. 1998 Mar 12;345(1):1-11. doi: 10.1016/s0014-2999(97)01604-x. Eur J Pharmacol. 1998. PMID: 9593588 Review.
-
Neuropeptide FF and modulation of pain.Brain Res. 1999 Nov 27;848(1-2):191-6. doi: 10.1016/s0006-8993(99)02044-2. Brain Res. 1999. PMID: 10612711 Review.
Cited by
-
Structural insights into the selective recognition of RF-amide peptides by neuropeptide FF receptor 2.EMBO Rep. 2025 May;26(9):2413-2434. doi: 10.1038/s44319-025-00428-2. Epub 2025 Mar 24. EMBO Rep. 2025. PMID: 40128413 Free PMC article.
-
Neuropeptide FF-related gene in fish (Larimichthys polyactis): identification, characterization, and potential anti-inflammatory function.Mol Biol Rep. 2022 Jul;49(7):6385-6394. doi: 10.1007/s11033-022-07447-5. Epub 2022 May 3. Mol Biol Rep. 2022. PMID: 35503491
-
Nerve Decompression Improves Spinal Synaptic Plasticity of Opioid Receptors for Pain Relief.Neurotox Res. 2018 Feb;33(2):362-376. doi: 10.1007/s12640-017-9799-5. Epub 2017 Aug 23. Neurotox Res. 2018. PMID: 28836121
-
BN-9, a chimeric peptide with mixed opioid and neuropeptide FF receptor agonistic properties, produces nontolerance-forming antinociception in mice.Br J Pharmacol. 2016 Jun;173(11):1864-80. doi: 10.1111/bph.13489. Epub 2016 Apr 21. Br J Pharmacol. 2016. PMID: 27018797 Free PMC article.
-
Dorsal hippocampal N-methyl-D-aspartate glutamatergic and δ-opioidergic systems modulate anxiety behaviors in rats in a noninteractive manner.Kaohsiung J Med Sci. 2011 Nov;27(11):485-93. doi: 10.1016/j.kjms.2011.06.011. Kaohsiung J Med Sci. 2011. PMID: 22005157 Free PMC article.
References
-
- ADVOKAT C., GULATI A. Spinal transection reduces both spinal antinociception and CNS concentration of systematically administered morphine in rats. Brain Res. 1991;555:251–258. - PubMed
-
- ADVOKAT C., RHEIN F.Q. Potentiation of morphine-induced antinociception in acute spinal rats by the NMDA antagonist dextrorphan. Brain Res. 1995;699:157–160. - PubMed
-
- ALLARD M., GEOFFRE S., LEGENDRE P., VINCENT J.D., SIMONNET G. Characterization of rat spinal cord receptors to FLFQPQRFarnide, a mammalian morphine modulating peptide: a binding study. Brain Res. 1989;500:169–176. - PubMed
-
- ALLARD M., JORDAN D., ZAJAC J.-M., RIS C., MARTIN D., MONKUANGO D., KOPP N., SIMONNET G. Autoradiography localization of receptors for neuropeptide FF, FLFQPQRFamide, in human spinal sensory system. Brain Res. 1994;633:127–132. - PubMed
-
- ALLARD M., THEODOSIS D.T., ROUSSELOT P., LOMBARD M.C., SIMONNET G. Characterization and localization of a putative morphine-modulating peptide, FLFQPQRFamide, in the rat spinal cord: biochemical and immunocytochemical studies. Neuroscience. 1991;49:81–92. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

