Anisatin modulation of the gamma-aminobutyric acid receptor-channel in rat dorsal root ganglion neurons
- PMID: 10455311
- PMCID: PMC1566146
- DOI: 10.1038/sj.bjp.0702700
Anisatin modulation of the gamma-aminobutyric acid receptor-channel in rat dorsal root ganglion neurons
Abstract
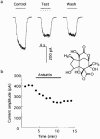
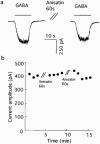
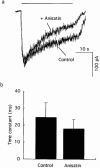
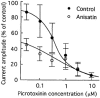
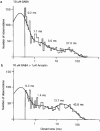
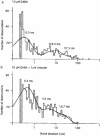
1. Anisatin, a toxic, insecticidally active component of Sikimi plant, is known to act on the GABA system. In order to elucidate the mechanism of anisatin interaction with the GABA system, whole-cell and single-channel patch clamp experiments were performed with rat dorsal root ganglion neurons in primary culture. 2. Repeated co-applications of GABA and anisatin suppressed GABA-induced whole-cell currents with an EC50 of 1.10 microM. No recovery of currents was observed after washout with anisatin-free solution. 3. However, pre-application of anisatin through the bath had no effect on GABA-induced currents. The decay phase of currents was accelerated by anisatin. These results indicate that anisatin suppression of GABA-induced currents requires opening of the channels and is use-dependent. 4. Anisatin suppression of GABA-induced currents was not voltage dependent. 5. Picrotoxinin attenuated anisatin suppression of GABA-induced currents. [3H]-EBOB binding to rat brain membranes was competitively inhibited by anisatin. These data indicated that anisatin bound to the picrotoxinin site. 6. At the single-channel level, anisatin did not alter the open time but prolonged the closed time. The burst duration was reduced and channel openings per burst were decreased indicating that anisatin decreased the probability of openings.
Figures





References
-
- ARAKAWA O., NAKAHIRO M., NARAHASHI T. Mercury modulation of GABA-activated chloride channels & non-specific cation channels in rat dorsal root ganglion neurons. Brain Res. 1991;551:58–63. - PubMed
-
- BERMUDEZ I., HAWKINS C.A., TAYLOR A.M., BEADLE D.J. Actions of insecticides on the insect GABA receptor complex. J. Receptor Res. 1991;11:221–232. - PubMed
-
- BLOOMQUIST J.R., ROUSH R.T., FFRENCH-CONSTANT R.H. Reduced neuronal sensitivity to dieldrin and picrotoxinin in a cyclodiene-resistant strain of Drosophila melanogaster (Meigen) Arch. Insect. Biochem. Physiol. 1992;19:17–25. - PubMed
-
- BURT D.R., KAMATCHI G.L. GABAA receptor subtypes: from pharmacology to molecular biology. FASEB J. 1991;4:2916–2923. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

