Calcium channels involved in the inhibition of acetylcholine release by presynaptic muscarinic receptors in rat striatum
- PMID: 10455319
- PMCID: PMC1566163
- DOI: 10.1038/sj.bjp.0702721
Calcium channels involved in the inhibition of acetylcholine release by presynaptic muscarinic receptors in rat striatum
Abstract
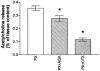
1. The mechanism of the inhibitory action of presynaptic muscarinic receptors on the release of acetylcholine from striatal cholinergic neurons is not known. We investigated how the electrically stimulated release of [3H]-acetylcholine from superfused rat striatal slices and its inhibition by carbachol are affected by specific inhibitors of voltage-operated calcium channels of the L-type (nifedipine), N-type (omega-conotoxin GVIA) and P/Q-type (omega-agatoxin IVA). 2. The evoked release of [3H]-acetylcholine was not diminished by nifedipine but was lowered by omega-conotoxin GVIA and by omega-agatoxin IVA, indicating that both the N- and the P/Q-type (but not the L-type) channels are involved in the release. The N-type channels were responsible for approximately two thirds of the release. The release was >97% blocked when both omega-toxins acted together. 3. The inhibition of [3H]-acetylcholine release by carbachol was not substantially affected by the blockade of the L- or P/Q-type channels. It was diminished but not eliminated by the blockade of the N-type channels. 4. In experiments on slices in which cholinesterases had been inhibited by paraoxon, inhibition of [3H]-acetylcholine release by endogenous acetylcholine accumulating in the tissue could be demonstrated by the enhancement of the release after the addition of atropine. The inhibition was higher in slices with functional N-type than with functional P/Q-type channels. 5. We conclude that both the N- and the P/Q-type calcium channels contribute to the stimulation-evoked release of acetylcholine in rat striatum, that the quantitative contribution of the N-type channels is higher, and that the inhibitory muscarinic receptors are more closely coupled with the N-type than with the P/Q-type calcium channels.
Figures




Similar articles
-
Presynaptic inhibition of synaptic transmission in the rat hippocampus by activation of muscarinic receptors: involvement of presynaptic calcium influx.Br J Pharmacol. 1997 Oct;122(3):511-9. doi: 10.1038/sj.bjp.0701400. Br J Pharmacol. 1997. PMID: 9351508 Free PMC article.
-
Control of glutamate release by calcium channels and kappa-opioid receptors in rodent and primate striatum.Br J Pharmacol. 1999 May;127(1):275-83. doi: 10.1038/sj.bjp.0702523. Br J Pharmacol. 1999. PMID: 10369483 Free PMC article.
-
Effects of the putative P-type calcium channel blocker, R,R-(-)-daurisoline on neurotransmitter release.Naunyn Schmiedebergs Arch Pharmacol. 1995 Dec;352(6):670-8. doi: 10.1007/BF00171327. Naunyn Schmiedebergs Arch Pharmacol. 1995. PMID: 9053740
-
Metabolism and trafficking of N-type voltage-operated calcium channels in neurosecretory cells.J Bioenerg Biomembr. 1998 Aug;30(4):399-407. doi: 10.1023/a:1021945907635. J Bioenerg Biomembr. 1998. PMID: 9758335 Review.
-
Modulation of acetylcholine release in human cortical slices: possible implications for Alzheimer's disease.Pharmacol Ther. 1997;74(3):333-47. doi: 10.1016/s0163-7258(97)00006-5. Pharmacol Ther. 1997. PMID: 9352588 Review.
Cited by
-
Chronic exposure of NG108-15 cells to amyloid beta peptide (A beta(1-42)) abolishes calcium influx via N-type calcium channels.Neurochem Res. 2001 Sep;26(8-9):1079-84. doi: 10.1023/a:1012361307306. Neurochem Res. 2001. PMID: 11699934
-
Natural Peptides in Drug Discovery Targeting Acetylcholinesterase.Molecules. 2018 Sep 13;23(9):2344. doi: 10.3390/molecules23092344. Molecules. 2018. PMID: 30217053 Free PMC article. Review.
-
Distinct synaptic mechanisms drive the behavioral response to acute stress and rapid correction by ketamine.Neuropsychopharmacology. 2024 Nov;49(12):1916-1924. doi: 10.1038/s41386-024-01908-0. Epub 2024 Jul 1. Neuropsychopharmacology. 2024. PMID: 38956176 Free PMC article.
-
Effectiveness of Intravenous and Nebulized MgSO4 in Children with Asthma Exacerbation: A Systematic Review and Meta-Analysis of Clinical Trials.Children (Basel). 2025 Aug 13;12(8):1064. doi: 10.3390/children12081064. Children (Basel). 2025. PMID: 40868515 Free PMC article. Review.
References
-
- ADAMS M.E., MYERS R.A., IMPERIAL J.S., OLIVERA B.M. Toxityping rat brain calcium channels with gamma-toxins from spider and cone snail venoms. Biochemistry. 1993;32:12566–12570. - PubMed
-
- BOEHM S., HUCK S. Inhibition of N-type calcium channels. The only mechanism by which presynaptic alpha(2)-autoreceptors control sympathetic transmitter release. Eur. J. Neurosci. 1996;8:1924–1931. - PubMed
-
- COUSIN M.A., HURST H., NICHOLLS D.G. Presynaptic calcium channels and field-evoked transmitter exocytosis from cultured cerebellar granule cells. Neuroscience. 1997;81:151–161. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

