Glucocorticoids potently block tumour necrosis factor-alpha- and lipopolysaccharide-induced apoptotic cell death in bovine glomerular endothelial cells upstream of caspase 3 activation
- PMID: 10455320
- PMCID: PMC1566164
- DOI: 10.1038/sj.bjp.0702726
Glucocorticoids potently block tumour necrosis factor-alpha- and lipopolysaccharide-induced apoptotic cell death in bovine glomerular endothelial cells upstream of caspase 3 activation
Abstract
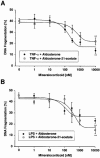
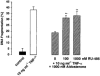
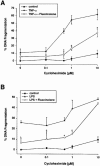
1. Endothelial cell damage in glomeruli and kidney arterioles appears to play a pivotal role in glomerular inflammatory diseases. Glomerular endothelial cells, a specialized microvascular cell type involved in the regulation of glomerular ultrafiltration, die by apoptosis in response to tumour necrosis factor-alpha (TNF-alpha), TNF-alpha/basic fibroblast growth factor (bFGF), TNF-alpha/cycloheximide, and bacterial lipopolysaccharide (LPS). Apoptotic cell death is characterized by extensive DNA cleavage, DNA ladder formation, and characteristic morphological alterations. 2. In search for apoptosis-preventing signals, we identified glucocorticoids as potent death preventing factors. Co-treatment of cells with 10 nM dexamethasone and TNF-alpha, TNF-alpha/bFGF, TNF-alpha/cycloheximide, or LPS blocked roughly 90% of apoptotic cell death in glomerular endothelial cells. 3. Similarly to dexamethasone (TNF-alpha- and LPS-induced apoptosis are prevented with IC50 values of 0.8 and 0.9 nM, respectively), other synthetic and natural forms of glucocorticoids, such as fluocinolone, prednisolone, hydrocortisone, and corticosterone potently inhibited cell death with IC50 values of 0.2, 6, 50 and 1000 nM, for TNF-alpha and 0.7, 8, 100 and 500 nM for LPS, respectively. 4. Apart from glucocorticoids, mineralocorticoids such as aldosterone also blocked TNF-alpha/LPS-induced apoptosis (IC50 approximately 500 nM for TNF-alpha and approximately 500 nM for LPS), whereas sex hormones, i. e. beta-estradiol and testosterone remained without effect. 5. The protective effect of glucocorticoids (and mineralocorticoids) required glucocorticoid receptor binding as it could be antagonized by the glucocorticoid receptor antagonist RU-486. Concerning TNF-alpha and LPS signal transduction, we found that dexamethasone efficiently prevented TNF-alpha- and LPS-induced activation of caspase-3-like proteases. Therefore, we postulate inhibitory mechanisms upstream of terminal death pathways.
Figures



Similar articles
-
Basic fibroblast growth factor selectively enhances TNF-alpha-induced apoptotic cell death in glomerular endothelial cells: effects on apoptotic signaling pathways.J Am Soc Nephrol. 2000 Dec;11(12):2199-2211. doi: 10.1681/ASN.V11122199. J Am Soc Nephrol. 2000. PMID: 11095643
-
Suppression of apoptosis by glucocorticoids in glomerular endothelial cells: effects on proapoptotic pathways.Br J Pharmacol. 2000 Apr;129(8):1673-83. doi: 10.1038/sj.bjp.0703255. Br J Pharmacol. 2000. PMID: 10780973 Free PMC article.
-
Tumor necrosis factor-alpha and lipopolysaccharide induce apoptotic cell death in bovine glomerular endothelial cells.Kidney Int. 1999 Jun;55(6):2322-37. doi: 10.1046/j.1523-1755.1999.00473.x. Kidney Int. 1999. PMID: 10354280
-
Dexamethasone inhibits TNF-alpha-induced apoptosis and IAP protein downregulation in MCF-7 cells.Br J Pharmacol. 2001 Jun;133(4):467-76. doi: 10.1038/sj.bjp.0704093. Br J Pharmacol. 2001. PMID: 11399663 Free PMC article.
-
Lipopolysaccharide induces disseminated endothelial apoptosis requiring ceramide generation.J Exp Med. 1997 Dec 1;186(11):1831-41. doi: 10.1084/jem.186.11.1831. J Exp Med. 1997. PMID: 9382882 Free PMC article.
Cited by
-
Randomized controlled studies on the efficacy of antiarthritic agents in inhibiting cartilage degeneration and pain associated with progression of osteoarthritis in the rat.Arthritis Res Ther. 2016 Jan 21;18:24. doi: 10.1186/s13075-016-0921-5. Arthritis Res Ther. 2016. PMID: 26794830 Free PMC article.
-
Resolvin D1 Protects Lipopolysaccharide-induced Acute Kidney Injury by Down-regulating Nuclear Factor-kappa B Signal and Inhibiting Apoptosis.Chin Med J (Engl). 2016 May 5;129(9):1100-7. doi: 10.4103/0366-6999.180517. Chin Med J (Engl). 2016. PMID: 27098797 Free PMC article.
-
Role of Endothelial Glucocorticoid Receptor in the Pathogenesis of Kidney Diseases.Int J Mol Sci. 2021 Dec 10;22(24):13295. doi: 10.3390/ijms222413295. Int J Mol Sci. 2021. PMID: 34948091 Free PMC article. Review.
-
Dexamethasone ameliorates renal ischemia-reperfusion injury.J Am Soc Nephrol. 2009 Nov;20(11):2412-25. doi: 10.1681/ASN.2008080868. Epub 2009 Sep 24. J Am Soc Nephrol. 2009. PMID: 19797168 Free PMC article.
-
Programmed Cell Death in Sepsis Associated Acute Kidney Injury.Front Med (Lausanne). 2022 May 17;9:883028. doi: 10.3389/fmed.2022.883028. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35655858 Free PMC article. Review.
References
-
- AUPHAN N., DIDONATO J.A., ROSETTE C., HELMBERG A., KARIN M. Immunosuppression by glucocorticoids: inhibition of NF-κB activity through induction of IκB synthesis. Science. 1995;270:286–290. - PubMed
-
- BALLERMANN B.J. Regulation of bovine glomerular endothelial cell growth in vitro. Am. J. Physiol. 1989;256:C182–C189. - PubMed
-
- BAUD L., FOUQUERAY B., PHILIPPE C., AMRANI A. Tumor necrosis factor alpha and mesangial cells. Kidney Int. 1992;41:600–603. - PubMed
-
- BAUD L., OUDINET J.P., BENS M., NOE L., PERALDI M.N., RONDEAU E., ETIENNE J., ARDAILLOU R. Production of tumor necrosis factor by rat mesangial cells in response to bacterial lipopolysaccharide. Kidney Int. 1989;35:1111–1118. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

