KATP channels and 'border zone' arrhythmias: role of the repolarization dispersion between normal and ischaemic ventricular regions
- PMID: 10455327
- PMCID: PMC1566150
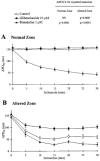
- DOI: 10.1038/sj.bjp.0702704
KATP channels and 'border zone' arrhythmias: role of the repolarization dispersion between normal and ischaemic ventricular regions
Abstract
1. In order to investigate the role of KATP channel activation and repolarization dispersion on the 'border zone' arrhythmias induced by ischaemia-reperfusion, the effects of glibenclamide and bimakalim, agents modifying action potential (AP) duration, were studied in an in vitro model of myocardial 'border zone'. 2. The electrophysiological effects of 10 microM glibenclamide and 1 microM bimakalim (n=8 each), respectively KATP channel blocker and activator, were investigated on guinea-pig ventricular strips submitted partly to normal conditions (normal zone, NZ) and partly to simulated ischaemic then reperfused conditions (altered zone, AZ). 3. By preventing the ischaemia-induced AP shortening (P<0.0001), glibenclamide reduced the dispersion of AP duration 90% (APD90) between NZ and AZ (P<0.0001), and concomitantly inhibited the 'border zone' arrhythmias induced by an extrastimulus (ES), their absence being significantly related to the lessened APD90 dispersion (chi2=8.28, P<0.01). 4. Bimakalim, which also reduced the APD90 dispersion (P<0.005) due to differential AP shortening in normal and ischaemic tissues, decreased the incidence of myocardial conduction blocks (25% of preparations versus 83% in control, n=12, P<0.05) and favoured 'border zone' spontaneous arrhythmias (75% of preparations versus 25% in control, P<0.05). 5. During reperfusion, unlike bimakalim, glibenclamide inhibited the ES-induced arrhythmias and reduced the incidence of the spontaneous ones (12% of preparations versus 92% in control, P<0.05), this latter effect being significantly related (chi2=6.13, P<0.02) to the lessened ischaemia-induced AP shortening in the presence of glibenclamide (P<0.0001). 6. These results suggest that KATP blockade may protect the ischaemic-reperfused myocardium from 'border zone' arrhythmias concomitantly with a reduction of APD90 dispersion between normal and ischaemic regions. Conversely, KATP channel activation may modify the incidence of conduction blocks and exacerbate the ischaemia-induced 'border zone' arrhythmias.
Figures



References
-
- AUCHAMPACH J.A., CAVERO I., GROSS G.J. Nicorandil attenuates myocardial dysfunction associated with transient ischemia by opening ATP-dependent potassium channels. J. Cardiovasc. Pharmacol. 1992;20:765–771. - PubMed
-
- BARRETT T.D., WALKER M.J.A. Glibenclamide does not prevent action potential shortening induced by ischemia in anesthetized rabbits but reduces ischemia-induced arrhythmias. J. Mol. Cell. Cardiol. 1998;30:999–1008. - PubMed
-
- BÉLICHARD P, , PRUNEAU D., ROUET R., SALZMANN J.L. Electrophysiological responses of hypertrophied rat myocardium to combined hypoxia, hyperkalemia and acidosis. J. Cardiovasc. Pharmacol. 1991;17:S141–S145. - PubMed
-
- BERNAUER W. Concerning the effect of the K+ channel blocking agent glibenclamide on ischaemic and reperfusion arrhythmias. Eur. J. Pharmacol. 1997;326:147–156. - PubMed
-
- BRIL A., LAVILLE M.P., GOUT B. Effects of glibenclamide on ventricular arrhythmias and cardiac function in ischaemia and reperfusion in isolated rat heart. Cardiovasc. Res. 1992;26:1069–1076. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

