Sulfated polysaccharide-directed recruitment of mammalian host proteins: a novel strategy in microbial pathogenesis
- PMID: 10456887
- PMCID: PMC96765
- DOI: 10.1128/IAI.67.9.4463-4468.1999
Sulfated polysaccharide-directed recruitment of mammalian host proteins: a novel strategy in microbial pathogenesis
Abstract
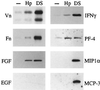
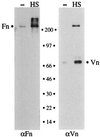
Fundamental to the virulence of microbial pathogens is their capacity for adaptation and survival within variable, and often hostile, environments encountered in the host. We describe a novel, extragenomic mechanism of surface modulation which may amplify the adaptive and pathogenic potential of numerous bacterial species, including Staphylococcus, Yersinia, and pathogenic Neisseria species, as well as Helicobacter pylori and Streptococcus pyogenes. The mechanism involves specific bacterial recruitment of heparin, glycosaminoglycans, or related sulfated polysaccharides, which in turn serve as universal binding sites for a diverse array of mammalian heparin binding proteins, including adhesive glycoproteins (vitronectin and fibronectin), inflammatory (MCP-3, PF-4, and MIP-1alpha) and immunomodulatory (gamma interferon) intermediates, and fibroblast growth factor. This strategy impacts key aspects of microbial pathogenicity as exemplified by increased bacterial invasion of epithelial cells and inhibition of chemokine-induced chemotaxis. Our findings illustrate a previously unrecognized form of parasitism that complements classical virulence strategies encoded within the microbial genome.
Figures
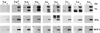
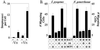

References
-
- Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines—CXC and CC chemokines. Adv Immunol. 1994;55:97–179. - PubMed
-
- Barnes D W, Reing J E, Amos B. Heparin-binding properties of human serum spreading factor. J Biol Chem. 1985;260:9117–9122. - PubMed
-
- Ben-Baruch A, Xu L, Young P, Bengali K, Oppenheim J, Wang J. Monocyte chemotactic protein-3 (MCP3) interacts with multiple leukocyte receptors. C-C CKR1, a receptor for macrophage inflammatory protein-1 alpha/Rantes, is also a functional receptor for MCP3. J Biol Chem. 1995;270:22123–22128. - PubMed
-
- Bodaghi B, Jones T R, Zipeto D, Vita C, Sun L, Laurent L, Arenzana-Seisdedos F, Virelizier J L, Michelson S. Chemokine sequestration by viral chemoreceptors as a novel viral escape strategy: withdrawal of chemokines from the environment of cytomegalovirus-infected cells. J Exp Med. 1998;188:855–866. - PMC - PubMed
-
- Cardin A D, Demeter D A, Weintraub H J, Jackson R L. Molecular design and modeling of protein-heparin interactions. Methods Enzymol. 1991;203:556–583. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

