Preliminary characterization of a Mycobacterium abscessus mutant in human and murine models of infection
- PMID: 10456919
- PMCID: PMC96797
- DOI: 10.1128/IAI.67.9.4700-4707.1999
Preliminary characterization of a Mycobacterium abscessus mutant in human and murine models of infection
Abstract
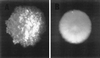
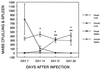
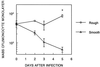
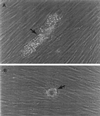
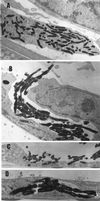
The ability to persist in the host after the establishment of infection is an important virulence determinant for mycobacteria. Mycobacterium abscessus is a rapidly growing mycobacterial species which causes a variety of clinical syndromes in humans. We have obtained a rough, wild-type human clinical isolate of M. abscessus (M. abscessus-R) and a smooth, attenuated mutant (M. abscessus-S) which spontaneously dissociated from the clinical isolate. We have found that M. abscessus-R is able to persist and multiply in a murine pulmonary infection model in contrast to M. abscessus-S, which is rapidly cleared. To understand the basis for this difference, we characterized the behavior of these variants in human tissue culture models of infection. M. abscessus-R is able to persist and multiply in human monocytes, while M. abscessus-S is deficient in this ability. Both of these variants are phagocytized by human monocytes. M. abscessus-R resides in a phagosome typical for pathogenic mycobacteria with a tightly adherent phagosomal membrane. In contrast, M. abscessus-S resides in a "loose" phagosome with the phagosomal membrane separated from the bacterial cell wall. Both M. abscessus variants also have distinctive growth patterns in a recently described fibroblast-mycobacterium microcolony assay, with M. abscessus-R exhibiting growth characteristics similar to those previously reported for virulent M. tuberculosis and M. abscessus-S exhibiting growth characteristics similar to those previously reported for avirulent M. tuberculosis. In both the monocyte infection assay and the murine pulmonary infection model, numerous infected mononuclear phagocyte aggregates develop at sites of M. abscessus-R infection, but are absent with M. abscessus-S infection. We conclude that a mutation has occurred in the M. abscessus-S variant which has altered the ability of this organism to persist and multiply in host cells and that this may be related to the phenotypic changes we have observed in our tissue culture models of infection.
Figures



References
-
- Beaman, B. Personal communication.
-
- Belisle J T, McNeil M R, Chatterjee D, Inamine J M, Brennan P J. Expression of the core lipopeptide of the glycopeptidolipid surface antigens in rough mutants of Mycobacterium avium. J Biol Chem. 1993;268:10510–10516. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

