Use of genetically manipulated strains of Clostridium perfringens reveals that both alpha-toxin and theta-toxin are required for vascular leukostasis to occur in experimental gas gangrene
- PMID: 10456947
- PMCID: PMC96825
- DOI: 10.1128/IAI.67.9.4902-4907.1999
Use of genetically manipulated strains of Clostridium perfringens reveals that both alpha-toxin and theta-toxin are required for vascular leukostasis to occur in experimental gas gangrene
Abstract
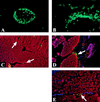
A hallmark of gas gangrene (clostridial myonecrosis) pathology is a paucity of leukocytes infiltrating the necrotic tissue. The cause of this paucity most likely relates to the observation of leukocyte aggregates at the border of the area of tissue necrosis, often within the microvasculature itself. Infecting mice with genetically manipulated strains of Clostridium perfringens type A (deficient in either alpha-toxin or theta-toxin production) resulted in significantly reduced leukocyte aggregation when alpha-toxin was absent and complete abrogation of leukocyte aggregation when theta-toxin was absent. Thus, both alpha-toxin and theta-toxin are necessary for the characteristic vascular leukostasis observed in clostridial myonecrosis.
Figures
References
-
- Awad M, Bryant A, Stevens D, Rood J. Virulence studies on chromosomal α-toxin and θ-toxin mutants constructed by allelic exchange provide genetic evidence for the essential role of α-toxin in Clostridium perfringens-mediated gas gangrene. Mol Microbiol. 1995;15:191–202. - PubMed
-
- Awad M, Rood J. Isolation of α-toxin, θ-toxin and κ-toxin mutants of Clostridium perfringens by Tn916 mutagenesis. Microb Pathog. 1997;22:275–284. - PubMed
-
- Bannam T, Rood J. Clostridium perfringens-Escherichia coli shuttle vectors that carry single antibiotic resistance determinants. Plasmid. 1992;229:233–235. - PubMed
-
- Bryant A, Bergstrom R, Zimmerman G, Salyer J, Hill H, Tweten R, Sato H, Stevens D. Clostridium perfringens invasiveness is enhanced by effects of theta toxin upon PMNL structure and function: the roles of leukocytotoxicity and expression of CD11/CD18 adherence glycoprotein. FEMS Immunol Med Microbiol. 1993;7:321–336. - PubMed
-
- Bryant A, Stevens D. Phospholipase C and perfringolysin O from Clostridium perfringens upregulate endothelial cell-leukocyte adherence molecule 1 and intercellular leukocyte adherence molecule 1 expression and induce interleukin-8 synthesis in cultured human umbilical vein endothelial cells. Infect Immun. 1996;64:358–362. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

