ATP-sensitive K+ channel openers prevent Ca2+ overload in rat cardiac mitochondria
- PMID: 10457054
- PMCID: PMC2269505
- DOI: 10.1111/j.1469-7793.1999.0347m.x
ATP-sensitive K+ channel openers prevent Ca2+ overload in rat cardiac mitochondria
Abstract
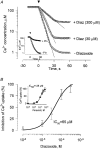
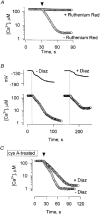
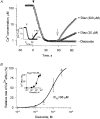
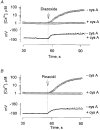
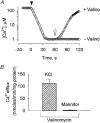
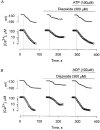
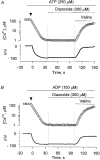
1. Mitochondrial dysfunction, secondary to excessive accumulation of Ca2+, has been implicated in cardiac injury. We here examined the action of potassium channel openers on mitochondrial Ca2+ homeostasis, as these cardioprotective ion channel modulators have recently been shown to target a mitochondrial ATP-sensitive K+ channel. 2. In isolated cardiac mitochondria, diazoxide and pinacidil decreased the rate and magnitude of Ca2+ uptake into the mitochondrial matrix with an IC50 of 65 and 128 microM, respectively. At all stages of Ca2+ uptake, the potassium channel openers depolarized the mitochondrial membrane thereby reducing Ca2+ influx through the potential-dependent mitochondrial uniporter. 3. Diazoxide and pinacidil, in a concentration-dependent manner, also activated release of Ca2+ from mitochondria. This was prevented by cyclosporin A, an inhibitor of Ca2+ release through the mitochondrial permeability transition pore. 4. Replacement of extramitochondrial K+ with mannitol abolished the effects of diazoxide and pinacidil on mitochondrial Ca2+, while the K+ ionophore valinomycin mimicked the effects of the potassium channel openers. 5. ATP and ADP, which block K+ flux through mitochondrial ATP-sensitive K+ channels, inhibited the effects of potassium channel openers, without preventing the action of valinomycin. 6. In intact cardiomyocytes, diazoxide also induced mitochondrial depolarization and decreased mitochondrial Ca2+ content. These effects were inhibited by the mitochondrial ATP-sensitive K+ channel blocker 5-hydroxydecanoic acid. 7. Thus, potassium channel openers prevent mitochondrial Ca2+ overload by reducing the driving force for Ca2+ uptake and by activating cyclosporin-sensitive Ca2+ release. In this regard, modulators of an ATP-sensitive mitochondrial K+ conductance may contribute to the maintenance of mitochondrial Ca2+ homeostasis.
Figures

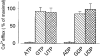

Similar articles
-
Potassium channel openers protect cardiac mitochondria by attenuating oxidant stress at reoxygenation.Am J Physiol Heart Circ Physiol. 2002 Feb;282(2):H531-9. doi: 10.1152/ajpheart.00552.2001. Am J Physiol Heart Circ Physiol. 2002. PMID: 11788400
-
Mitochondrial ATP-sensitive potassium channel activation protects cerebellar granule neurons from apoptosis induced by oxidative stress.Stroke. 2003 Jul;34(7):1796-802. doi: 10.1161/01.STR.0000077017.60947.AE. Epub 2003 Jun 5. Stroke. 2003. PMID: 12791941
-
Adenine nucleotide translocase mediates the K(ATP)-channel-openers-induced proton and potassium flux to the mitochondrial matrix.J Bioenerg Biomembr. 2003 Apr;35(2):141-8. doi: 10.1023/a:1023746103401. J Bioenerg Biomembr. 2003. PMID: 12887012
-
ATP-sensitive K+ channel openers: old drugs with new clinical benefits for the heart.Curr Vasc Pharmacol. 2003 Oct;1(3):251-8. doi: 10.2174/1570161033476646. Curr Vasc Pharmacol. 2003. PMID: 15320472 Review.
-
Multidimensional Regulation of Cardiac Mitochondrial Potassium Channels.Cells. 2021 Jun 19;10(6):1554. doi: 10.3390/cells10061554. Cells. 2021. PMID: 34205420 Free PMC article. Review.
Cited by
-
Pharmacological plasticity of cardiac ATP-sensitive potassium channels toward diazoxide revealed by ADP.Proc Natl Acad Sci U S A. 1999 Oct 12;96(21):12162-7. doi: 10.1073/pnas.96.21.12162. Proc Natl Acad Sci U S A. 1999. PMID: 10518593 Free PMC article.
-
Mitochondrial ATP-sensitive K+ channels, protectors of the heart.J Physiol. 2010 Jan 15;588(Pt 2):283-6. doi: 10.1113/jphysiol.2009.179028. J Physiol. 2010. PMID: 20080515 Free PMC article. No abstract available.
-
Diazoxide, a K(ATP) channel opener, prevents ischemia-reperfusion injury in rodent pancreatic islets.Cell Transplant. 2015;24(1):25-36. doi: 10.3727/096368913X673441. Epub 2013 Sep 10. Cell Transplant. 2015. PMID: 24070013 Free PMC article.
-
Myocardial Hsp70 phosphorylation and PKC-mediated cardioprotection following exercise.Cell Stress Chaperones. 2009 Mar;14(2):141-50. doi: 10.1007/s12192-008-0065-x. Epub 2008 Jul 31. Cell Stress Chaperones. 2009. PMID: 18668351 Free PMC article.
-
Cardiac subsarcolemmal and interfibrillar mitochondria display distinct responsiveness to protection by diazoxide.PLoS One. 2012;7(9):e44667. doi: 10.1371/journal.pone.0044667. Epub 2012 Sep 4. PLoS One. 2012. PMID: 22973464 Free PMC article.
References
-
- Beavis AD, Lu Y, Garlid KD. On the regulation of K+ uniport in intact mitochondria by adenine nucleotides and nucleotide analogs. Journal of Biological Chemistry. 1993;268:997–1004. - PubMed
-
- Belyaeva E, Szewczyk A, Mikolaek B, Nalecz M, Wojtczak L. Demonstration of glibenclamide-sensitive K+ fluxes in rat liver mitochondria. Biochemistry and Molecular Biology International. 1993;31:493–500. - PubMed
-
- Bernardi P, Petronilli V. The permeability transition pore as a mitochondrial Ca2+ release channel: A critical appraisal. Journal of Bioenergetics and Biomembranes. 1996;28:131–138. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

