Activity-dependent modulation of K+ currents at presynaptic terminals of mammalian central synapses
- PMID: 10457060
- PMCID: PMC2269500
- DOI: 10.1111/j.1469-7793.1999.0427m.x
Activity-dependent modulation of K+ currents at presynaptic terminals of mammalian central synapses
Abstract
1. The activity-dependent regulation of presynaptic K+ currents at the CA3-CA1 synapse in the rat hippocampus was investigated during a train of evoked afferent action potentials. The waveforms of presynaptic compound action potentials (cAPs) and presynaptic Ca2+ transients ([Ca2+]pre,t) were measured with fluorescent voltage-sensitive and Ca2+-sensitive indicators in rat brain slices. 2. Under control conditions, presynaptic cAPs and the accompanying [Ca2+]pre,t displayed similar amplitudes for each stimulus, suggesting that there was no cumulative change of K+ and Ca2+ currents during the test train. However, when a subgroup of presynaptic K+ channels was blocked by a low concentration of 4-aminopyridine (4-AP, 40 microM), a significant facilitation of the [Ca2+]pre,t was observed. 3. This phenomenon was not due to a direct action of 4-AP on presynaptic Ca2+ channels, but to cumulative suppression of the K+ conductance as indicated by the corresponding change in waveforms of the cAP and presynaptic fibre volley. The observed facilitation was not an artifact by virtue of increased fibre recruitment, nor was it related to the accumulation of extracellular K+; rather, it was dependent on Ca2+ influx and stimulation frequency. The time course of recovery from facilitation was closely related to the decay of the intracellular Ca2+ concentration. 4. The facilitation was not blocked by a saturating concentration of 4-AP (8 mM) but was reduced during the application of the K+ channel blocker tetraethylammonium (TEA, 10 mM), implicating the involvement of TEA-sensitive K+ channels. Such activity-dependent suppression of presynaptic K+ conductance could lead to excessive transmitter release and might explain the hippocampal epileptiform activity that can be induced by application of 4-AP.
Figures
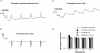

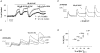
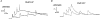

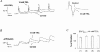


Similar articles
-
Modulation of transmitter release by action potential duration at the hippocampal CA3-CA1 synapse.J Neurophysiol. 1999 Jan;81(1):288-98. doi: 10.1152/jn.1999.81.1.288. J Neurophysiol. 1999. PMID: 9914289
-
K+ and Ca2+ channel blockers may enhance or depress sympathetic transmitter release via a Ca(2+)-dependent mechanism "upstream" of the release site.Neuroscience. 1991;44(3):673-92. doi: 10.1016/0306-4522(91)90087-5. Neuroscience. 1991. PMID: 1661385
-
Regulation of transmitter release at the squid giant synapse by presynaptic delayed rectifier potassium current.J Physiol. 1990 Dec;431:343-64. doi: 10.1113/jphysiol.1990.sp018333. J Physiol. 1990. PMID: 1983120 Free PMC article.
-
Action potential repolarization and a fast after-hyperpolarization in rat hippocampal pyramidal cells.J Physiol. 1987 Apr;385:733-59. doi: 10.1113/jphysiol.1987.sp016517. J Physiol. 1987. PMID: 2443676 Free PMC article. Review.
-
Presynaptic calcium channels: pharmacology and regulation.Neurochem Int. 1995 Jun;26(6):539-58. doi: 10.1016/0197-0186(94)00149-o. Neurochem Int. 1995. PMID: 7670358 Review.
Cited by
-
KV1 and KV3 Potassium Channels Identified at Presynaptic Terminals of the Corticostriatal Synapses in Rat.Neural Plast. 2016;2016:8782518. doi: 10.1155/2016/8782518. Epub 2016 Jun 9. Neural Plast. 2016. PMID: 27379187 Free PMC article.
-
Axonal noise as a source of synaptic variability.PLoS Comput Biol. 2014 May 8;10(5):e1003615. doi: 10.1371/journal.pcbi.1003615. eCollection 2014 May. PLoS Comput Biol. 2014. PMID: 24809823 Free PMC article.
-
Degeneracy in the regulation of short-term plasticity and synaptic filtering by presynaptic mechanisms.J Physiol. 2017 Apr 15;595(8):2611-2637. doi: 10.1113/JP273482. Epub 2017 Feb 1. J Physiol. 2017. PMID: 28026868 Free PMC article.
-
The potassium channel subunit Kvβ1 serves as a major control point for synaptic facilitation.Proc Natl Acad Sci U S A. 2020 Nov 24;117(47):29937-29947. doi: 10.1073/pnas.2000790117. Epub 2020 Nov 9. Proc Natl Acad Sci U S A. 2020. PMID: 33168717 Free PMC article.
-
The Mechanisms and Functions of Synaptic Facilitation.Neuron. 2017 May 3;94(3):447-464. doi: 10.1016/j.neuron.2017.02.047. Neuron. 2017. PMID: 28472650 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

