Enhanced antigen-presenting activity and tumour necrosis factor-alpha-independent activation of dendritic cells following treatment with Mycobacterium bovis bacillus Calmette-Guérin
- PMID: 10457216
- PMCID: PMC2326884
- DOI: 10.1046/j.1365-2567.1999.00818.x
Enhanced antigen-presenting activity and tumour necrosis factor-alpha-independent activation of dendritic cells following treatment with Mycobacterium bovis bacillus Calmette-Guérin
Abstract
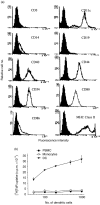
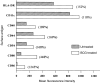
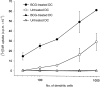
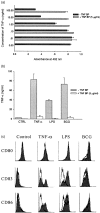
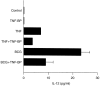
Dendritic cells (DCs) are most potent among the antigen-presenting cells and are believed to be crucial for the initiation of a primary T-cell response to foreign antigens. Mycobacterial infection within macrophages is controlled by cell-mediated immunity. To elucidate the stimulation of immune response by Mycobacterium bovis bacillus Calmette-Guérin (BCG), we purified DCs from precursor cells in human peripheral blood mononuclear cells (PBMC) by culturing them with granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4) and characterized their surface antigen expression. The interaction of cultured DCs with BCG resulted in increased surface expression of several DC-related marker antigens. BCG also induced reduction of endocytosis, enhancement of CD83 expression as well as B7 costimulatory molecules and IL-12 production, suggesting that BCG treatment directly induces DCs to mature. BCG-treated DCs were much more potent antigen-presenting cells in allogeneic immune response than untreated DCs. Moreover, while the neutralization of tumour necrosis factor-alpha (TNF-alpha) significantly blocked the DC maturation induced by lipopolysaccharide (LPS), it could not inhibit the induction of DC maturation by the BCG treatment, indicating that TNF-alpha production plays a minor role in the BCG-induced DC maturation. However, the neutralization of TNF-alpha resulted in decreased IL-12 production by activated DCs. These results suggest that infection with BCG might evoke direct activation and maturation of DC and the general immune stimulant effect of BCG might be related with the activation of DCs.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

