Analysis of CD44-containing lipid rafts: Recruitment of annexin II and stabilization by the actin cytoskeleton
- PMID: 10459018
- PMCID: PMC2156143
- DOI: 10.1083/jcb.146.4.843
Analysis of CD44-containing lipid rafts: Recruitment of annexin II and stabilization by the actin cytoskeleton
Abstract
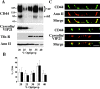
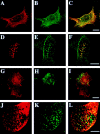
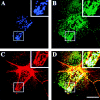
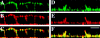
CD44, the major cell surface receptor for hyaluronic acid (HA), was shown to localize to detergent-resistant cholesterol-rich microdomains, called lipid rafts, in fibroblasts and blood cells. Here, we have investigated the molecular environment of CD44 within the plane of the basolateral membrane of polarized mammary epithelial cells. We show that CD44 partitions into lipid rafts that contain annexin II at their cytoplasmic face. Both CD44 and annexin II were released from these lipid rafts by sequestration of plasma membrane cholesterol. Partition of annexin II and CD44 to the same type of lipid rafts was demonstrated by cross-linking experiments in living cells. First, when CD44 was clustered at the cell surface by anti-CD44 antibodies, annexin II was recruited into the cytoplasmic leaflet of CD44 clusters. Second, the formation of intracellular, submembranous annexin II-p11 aggregates caused by expression of a trans-dominant mutant of annexin II resulted in coclustering of CD44. Moreover, a frequent redirection of actin bundles to these clusters was observed. These basolateral CD44/annexin II-lipid raft complexes were stabilized by addition of GTPgammaS or phalloidin in a semipermeabilized and cholesterol-depleted cell system. The low lateral mobility of CD44 in the plasma membrane, as assessed with fluorescent recovery after photobleaching (FRAP), was dependent on the presence of plasma membrane cholesterol and an intact actin cytoskeleton. Disruption of the actin cytoskeleton dramatically increased the fraction of CD44 which could be recovered from the light detergent-insoluble membrane fraction. Taken together, our data indicate that in mammary epithelial cells the vast majority of CD44 interacts with annexin II in lipid rafts in a cholesterol-dependent manner. These CD44-containing lipid microdomains interact with the underlying actin cytoskeleton.
Figures


References
-
- Bellagamba C., Hubaishy I., Bjorge J.D., Fitzpatrick S.L., Fujita D.J., Waisman D. Tyrosine phosphorylation of annexin II tetramer is stimulated by membrane binding. J. Biol. Chem. 1997;272:3195–3199. - PubMed
-
- Bourguignon L., Zhu D., Zhu H. CD44 isoform-cytoskeleton interaction in oncogenic signaling and tumor progression. Front. Biosci. 1998;3:637–649. - PubMed
-
- Brown D.A., London E. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 1998;14:111–136. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

