The insulin receptor tyrosine kinase substrate p58/53 and the insulin receptor are components of CNS synapses
- PMID: 10460236
- PMCID: PMC6782521
- DOI: 10.1523/JNEUROSCI.19-17-07300.1999
The insulin receptor tyrosine kinase substrate p58/53 and the insulin receptor are components of CNS synapses
Abstract
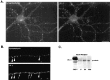
The synapse is the primary locus of cell-cell communication in the nervous system. It is now clear that the synapse incorporates diverse cell signaling modalities in addition to classical neurotransmission. Here we show that two components of the insulin pathway are localized at CNS synapses, where they are components of the postsynaptic density (PSD). An immunochemical screen revealed that polypeptides of 58 and 53 kDa (p58/53) were highly enriched in PSD fractions from rat cerebral cortex, hippocampus, and cerebellum. These polypeptides were purified and microsequenced, revealing that p58/53 is identical to the insulin receptor tyrosine kinase substrate p58/53 (IRSp53). Our analysis of IRSp58/53 mRNA suggests that within rat brain there is one coding region for IRSp58 and IRSp53; we find no evidence of alternative splicing. We demonstrate that IRSp58/53 is expressed in the synapse-rich molecular layer of the cerebellum and is highly concentrated at the synapses of cultured hippocampal neurons, where it co-localizes with the insulin receptor. Together, these data suggest that insulin signaling may play a role at CNS synapses.
Figures
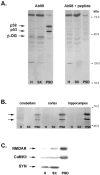

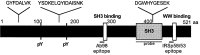

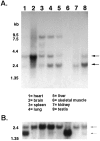
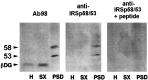

References
-
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
-
- Anai M, Ono H, Funaki M, Fukushima Y, Inukai K, Ogihara T, Sakoda H, Onishi Y, Yazaki Y, Kikuchi M, Oka Y, Asano T. Different subcellular distribution and regulation of expression of insulin receptor substrate (IRS)-3 from those of IRS-1 and IRS-2. J Biol Chem. 1998;273:29686–29692. - PubMed
-
- Baltensperger K, Karoor V, Paul H, Ruoho A, Czech MP, Malbon CC. The beta-adrenergic receptor is a substrate for the insulin receptor tyrosine kinase. J Biol Chem. 1996;271:1061–1064. - PubMed
-
- Baskin DG, Schwartz MW, Sipols AJ, D’Alessio DA, Goldstein BJ, White MF. Insulin receptor substrate-1 (IRS-1) expression in rat brain. Endocrinology. 1994;134:1952–1955. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
