Mechanisms underlying spontaneous oscillation and rhythmic firing in rat subthalamic neurons
- PMID: 10460267
- PMCID: PMC6782508
- DOI: 10.1523/JNEUROSCI.19-17-07617.1999
Mechanisms underlying spontaneous oscillation and rhythmic firing in rat subthalamic neurons
Abstract
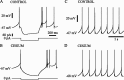
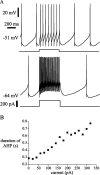
Subthalamic neurons drive basal ganglia output neurons in resting animals and relay cortical and thalamic activity to the same output neurons during movement. The first objective of this study was to determine the mechanisms underlying the spontaneous activity of subthalamic neurons in vitro and to gain insight into their resting discharge in vivo. The second objective was to determine the response of subthalamic neurons to depolarizing current injection and how intrinsic properties may shape their response to cortical and thalamic inputs during movement. Cell-attached and whole-cell recordings were made from subthalamic neurons in brain slices prepared from 3- to 4-week-old rats. The slow, rhythmic discharge of subthalamic neurons was resistant to blockade of excitatory synaptic transmission indicating that intrinsic currents underlie their spontaneous discharge. A persistent sodium current was the source of current during the depolarizing phase of the oscillation. A powerful afterhyperpolarization following each action potential was sufficient to terminate the depolarization. A long duration component of the spike afterhyperpolarization determined the period of the oscillation and was generated by an apamin-sensitive calcium-activated potassium current. Calcium entry responsible for that current was associated with action potentials. Subthalamic neurons exhibited a sigmoidal frequency-current relationship with the steeper portion starting at approximately 30-40 Hz. This property makes subthalamic neurons more sensitive to input at high firing rates associated with movement than at low rates associated with rest. We propose that the subthreshold persistent sodium current overcomes calcium activated potassium current which accumulates during high frequency firing and underlies the enhanced sensitivity to current >30 Hz.
Figures





References
-
- Benazzouz A, Gross C, Feger J, Boraud T, Bioulac B. Reversal of rigidity and improvement in motor performance by subthalamic high-frequency stimulation in MPTP-treated monkeys. Eur J Neurosci. 1993;5:382–389. - PubMed
-
- Benazzouz A, Boraud T, Feger J, Burbaud P, Bioulac B, Gross C. Alleviation of experimental hemiparkinsonism by high-frequency stimulation of the subthalamic nucleus in primates: a comparison with L-Dopa treatment. Mov Disord. 1996;11:627–632. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
