An autoregulatory circuit affecting peptide signaling in Bacillus subtilis
- PMID: 10464187
- PMCID: PMC94022
- DOI: 10.1128/JB.181.17.5193-5200.1999
An autoregulatory circuit affecting peptide signaling in Bacillus subtilis
Abstract
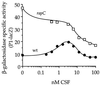
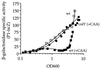
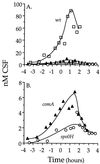
The competence and sporulation factor (CSF) of Bacillus subtilis is an extracellular pentapeptide produced from the product of phrC. CSF has at least three activities: (i) at low concentrations, it stimulates expression of genes activated by the transcription factor ComA; at higher concentrations, it (ii) inhibits expression of those same genes and (iii) stimulates sporulation. Because the activities of CSF are concentration dependent, we measured the amount of extracellular CSF produced by cells. We found that by mid-exponential phase, CSF accumulated to concentrations (1 to 5 nM) that stimulate ComA-dependent gene expression. Upon entry into stationary phase, CSF reached 50 to 100 nM, concentrations that stimulate sporulation and inhibit ComA-dependent gene expression. Transcription of phrC was found to be controlled by two promoters: P1, which precedes rapC, the gene upstream of phrC; and P2, which directs transcription of phrC only. Both RapC and CSF were found to be part of autoregulatory loops that affect transcription from P1, which we show is activated by ComA approximately P. RapC negatively regulates its own expression, presumably due to its ability to inhibit accumulation of ComA approximately P. CSF positively regulates its own expression, presumably due to its ability to inhibit RapC activity. Transcription from P2, which is controlled by the alternate sigma factor sigma(H), increased as cells entered stationary phase, contributing to the increase in extracellular CSF at this time. In addition to controlling transcription of phrC, sigmaH appears to control expression of at least one other gene required for production of CSF.
Figures





References
-
- Burbulys D, Trach K A, Hoch J A. Initiation of sporulation in B. subtilis is controlled by a multicomponent phosphorelay. Cell. 1991;64:545–552. - PubMed
-
- Carter H L, III, Tatti K M, Moran C P., Jr Cloning of a promoter used by sigma-H RNA polymerase in Bacillus subtilis. Gene. 1991;96:101–105. - PubMed
-
- Grossman A D. Genetic networks controlling the initiation of sporulation and the development of genetic competence in Bacillus subtilis. Annu Rev Genet. 1995;29:477–508. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

