The Escherichia coli NadR regulator is endowed with nicotinamide mononucleotide adenylyltransferase activity
- PMID: 10464228
- PMCID: PMC94063
- DOI: 10.1128/JB.181.17.5509-5511.1999
The Escherichia coli NadR regulator is endowed with nicotinamide mononucleotide adenylyltransferase activity
Abstract
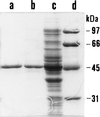
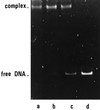
The first identification and characterization of a catalytic activity associated with NadR protein is reported. A computer-aided search for sequence similarity revealed the presence in NadR of a 29-residue region highly conserved among known nicotinamide mononucleotide adenylyltransferases. The Escherichia coli nadR gene was cloned into a T7-based vector and overexpressed. In addition to functionally specific DNA binding properties, the homogeneous recombinant protein catalyzes NAD synthesis from nicotinamide mononucleotide and ATP.
Figures
References
-
- Dahmen W, Webb B, Press J. The deamidodiphosphopyridine nucleotide and diphosphopyridine nucleotide pyrophosphorylases from Escherichia coli and yeast. Arch Biochem Biophys. 1967;120:440–450. - PubMed
-
- Emanuelli M, Natalini P, Raffaelli N, Ruggieri S, Vita A, Magni G. NAD biosynthesis in human placenta: purification and characterization of homogeneous NMN adenylyltransferase. Arch Biochem Biophys. 1992;298:29–34. - PubMed
-
- Foster J W, Penfound T. The bifunctional nadR regulator of Salmonella typhimurium: location of regions involved with DNA binding, nucleotide transport and intramolecular communication. FEMS Microbiol Lett. 1993;112:179–184. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

