Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation
- PMID: 10465785
- PMCID: PMC316949
- DOI: 10.1101/gad.13.16.2072
Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation
Erratum in
- Genes Dev 1999 Oct 1;13(19):2617
Abstract
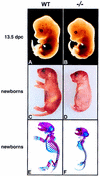
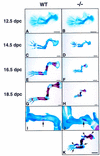
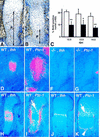
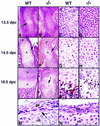
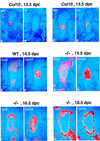
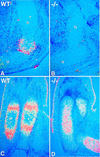
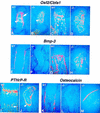
The mechanisms that control cell proliferation and cell differentiation during morphogenesis of the endochondral skeleton of vertebrates are poorly understood. Indian hedgehog (Ihh) signaling from prehypertrophic chondrocytes has been implicated in the control of chondrocyte maturation by way of feedback control of a second secreted factor parathyroid hormone-related peptide (PTHrP) at the articular surfaces. Analysis of an Ihh null mutant suggests a more extensive role for Ihh in skeletal development. Mutants display markedly reduced chondrocyte proliferation, maturation of chondrocytes at inappropriate position, and a failure of osteoblast development in endochondral bones. Together, the results suggest a model in which Ihh coordinates diverse aspects of skeletal morphogenesis through PTHrP-dependent and independent processes.
Figures

References
-
- Akiyama H, Shigeno C, Iyama K, Ito H, Hiraki Y, Konoshi J, Nakamura T. Indian hedgehog in the late-phase differentiation in mouse chondrogenic EC cells, ATDC5: Upregulation of type X collagen and osteoprotegerin ligand mRNAs. Biochem Biophys Res Commun. 1999;257:814–820. - PubMed
-
- Amizuka N, Henderson J E, Hoshi K, Warshawsky H, Ozawa H, Goltzman D, Karaplis AC. Programmed cell death of chondrocytes and aberrant chondrogenesis in mice homozygous for parathyroid hormone-related peptide gene deletion. Endocrinology. 1996;137:5055–5067. - PubMed
-
- Apte SS, Seldin MF, Hayashi M, Olsen BR. Cloning of the human and mouse type X collagen genes and mapping of the mouse type X collagen gene to chromosome 10. Eur J Biochem. 1992;206:217–224. - PubMed
-
- Aubin J, Liu F. The osteoblast lineage. In: Bilezikian JP, Raisz LG, Rodan GA, editors. Principles of bone biology. New York, NY: Academic Press; 1996. pp. 51–67.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
