The maximal affinity of ligands
- PMID: 10468550
- PMCID: PMC17830
- DOI: 10.1073/pnas.96.18.9997
The maximal affinity of ligands
Abstract
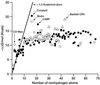
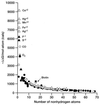
We explore the question of what are the best ligands for macromolecular targets. A survey of experimental data on a large number of the strongest-binding ligands indicates that the free energy of binding increases with the number of nonhydrogen atoms with an initial slope of approximately -1.5 kcal/mol (1 cal = 4.18 J) per atom. For ligands that contain more than 15 nonhydrogen atoms, the free energy of binding increases very little with relative molecular mass. This nonlinearity is largely ascribed to nonthermodynamic factors. An analysis of the dominant interactions suggests that van der Waals interactions and hydrophobic effects provide a reasonable basis for understanding binding affinities across the entire set of ligands. Interesting outliers that bind unusually strongly on a per atom basis include metal ions, covalently attached ligands, and a few well known complexes such as biotin-avidin.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

