The hsp70-associating protein Hap46 binds to DNA and stimulates transcription
- PMID: 10468585
- PMCID: PMC17865
- DOI: 10.1073/pnas.96.18.10194
The hsp70-associating protein Hap46 binds to DNA and stimulates transcription
Abstract
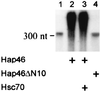
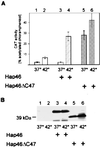
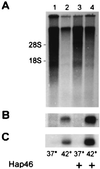
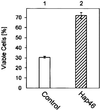
We investigated the ubiquitously expressed hsp70-associating protein Hap46, which is also called RAP46 and is homologous to BAG-1, for activities independent of hsp70 interactions. We observed in vitro binding to various DNA fragments but detected no apparent sequence specificity. Deletion of the amino-terminal decapeptide, which contains two clusters of three basic amino acids each, abolished the DNA-binding ability of Hap46. Similarly, exchange of either of these positively charged clusters for three alanines resulted in loss of DNA binding. Using a fusion of Hap46 and green fluorescent protein, we found preferential accumulation in cell nuclei on heat stress as compared with unstressed cells. The repressive effect of heat shock on overall transcriptional activity in human DU145 carcinoma cells was largely prevented when Hap46 was overexpressed by transfection. Such overproduction of Hap46 also resulted in enhanced expression of specific reporter gene constructs and in increased levels of mRNAs specific for hsp70 and hsp40 after temperature stress. In vitro transcription with nuclear extracts was stimulated greatly by Hap46. Like DNA binding, transcriptional enhancement required amino-terminally located basic amino acid residues but not the carboxyl-terminal portion of Hap46 known to participate in hsp70 interaction. Our results show that Hap46 is a bifunctional protein that can interact with both hsp70s and DNA, employing different portions of the molecule. They also suggest that Hap46 is involved in temperature-sensitive regulation of transcription, acting as a general transcriptional activator.
Figures
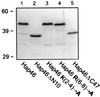

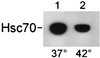
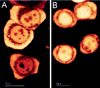
References
-
- Takayama S, Sato T, Krajewski S, Kochel K, Irie S, Millan J A, Reed J C. Cell. 1995;80:279–284. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

