Correction of the UDP-glucuronosyltransferase gene defect in the gunn rat model of crigler-najjar syndrome type I with a chimeric oligonucleotide
- PMID: 10468611
- PMCID: PMC17891
- DOI: 10.1073/pnas.96.18.10349
Correction of the UDP-glucuronosyltransferase gene defect in the gunn rat model of crigler-najjar syndrome type I with a chimeric oligonucleotide
Abstract
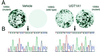
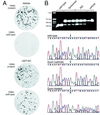
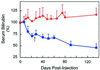
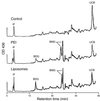
Crigler-Najjar syndrome type I is characterized by unconjugated hyperbilirubinemia resulting from an autosomal recessive inherited deficiency of hepatic UDP-glucuronosyltransferase (UGT) 1A1 activity. The enzyme is essential for glucuronidation and biliary excretion of bilirubin, and its absence can be fatal. The Gunn rat is an excellent animal model of this disease, exhibiting a single guanosine (G) base deletion within the UGT1A1 gene. The defect results in a frameshift and a premature stop codon, absence of enzyme activity, and hyperbilirubinemia. Here, we show permanent correction of the UGT1A1 genetic defect in Gunn rat liver with site-specific replacement of the absent G residue at nucleotide 1206 by using an RNA/DNA oligonucleotide designed to promote endogenous repair of genomic DNA. The chimeric oligonucleotide was either complexed with polyethylenimine or encapsulated in anionic liposomes, administered i.v., and targeted to the hepatocyte via the asialoglycoprotein receptor. G insertion was determined by PCR amplification, colony lift hybridizations, restriction endonuclease digestion, and DNA sequencing, and confirmed by genomic Southern blot analysis. DNA repair was specific, efficient, stable throughout the 6-month observation period, and associated with reduction of serum bilirubin levels. Our results indicate that correction of the UGT1A1 genetic lesion in the Gunn rat restores enzyme expression and bilirubin conjugating activity, with consequent improvement in the metabolic abnormality.
Figures



References
-
- Bosma P J, Seppen J, Goldhoorn B, Bakker C, Oude Elferink R P J, Roy Chowdhury J, Roy Chowdhury N, Jansen P L M. J Biol Chem. 1994;269:17960–17964. - PubMed
-
- Crigler J F, Najjar V A. Pediatrics. 1952;10:169–172. - PubMed
-
- Roy Chowdhury J, Wolkoff A W, Roy Chowdhury N, Arias I M. In: The Metabolic and Molecular Bases of Inherited Disease. Scriver C R, Beaudet A L, Sly W S, Valle D, editors. New York: McGraw-Hill; 1995. pp. 2161–2208.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

