Long-term expression of human coagulation factor VIII and correction of hemophilia A after in vivo retroviral gene transfer in factor VIII-deficient mice
- PMID: 10468616
- PMCID: PMC17896
- DOI: 10.1073/pnas.96.18.10379
Long-term expression of human coagulation factor VIII and correction of hemophilia A after in vivo retroviral gene transfer in factor VIII-deficient mice
Abstract
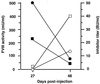
Hemophilia A is caused by a deficiency in coagulation factor VIII (FVIII) and predisposes to spontaneous bleeding that can be life-threatening or lead to chronic disabilities. It is well suited for gene therapy because a moderate increase in plasma FVIII concentration has therapeutic effects. Improved retroviral vectors expressing high levels of human FVIII were pseudotyped with the vesicular stomatitis virus G glycoprotein, were concentrated to high-titers (10(9)-10(10) colony-forming units/ml), and were injected intravenously into newborn, FVIII-deficient mice. High-levels (>/=200 milliunits/ml) of functional human FVIII production could be detected in 6 of the 13 animals, 4 of which expressed physiologic or higher levels (500-12,500 milliunits/ml). Five of the six expressers produced FVIII and survived an otherwise lethal tail-clipping, demonstrating phenotypic correction of the bleeding disorder. FVIII expression was sustained for >14 months. Gene transfer occurred into liver, spleen, and lungs with predominant FVIII mRNA expression in the liver. Six of the seven animals with transient or no detectable human FVIII developed FVIII inhibitors (7-350 Bethesda units/ml). These findings indicate that a genetic disease can be corrected by in vivo gene therapy using retroviral vectors.
Figures

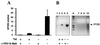



Comment in
-
Gene therapy for the hemophilias.Proc Natl Acad Sci U S A. 1999 Aug 31;96(18):9973-5. doi: 10.1073/pnas.96.18.9973. Proc Natl Acad Sci U S A. 1999. PMID: 10468539 Free PMC article. Review. No abstract available.
References
-
- Connelly S, Kaleko M. Thromb Haemostasis. 1997;78:31–36. - PubMed
-
- Chuah M K, Collen D, VandenDriessche T. Crit Rev Oncol Hematol. 1998;28:153–171. - PubMed
-
- Koster T, Blann A D, Briet E, Vandenbroucke J P, Roosendael F R. Lancet. 1995;345:152–155. - PubMed
-
- Chuah M K, VandenDriessche T, Morgan R A. Hum Gene Ther. 1995;6:1363–1377. - PubMed
-
- Hoeben R C, van der Jagt R C, Schoute F, van Tilburg N H, Verbeet M P, Briet E, van Ormondt H, van der Eb A J. J Biol Chem. 1990;265:7318–7323. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

