Bacillus subtilis aconitase is an RNA-binding protein
- PMID: 10468622
- PMCID: PMC17902
- DOI: 10.1073/pnas.96.18.10412
Bacillus subtilis aconitase is an RNA-binding protein
Abstract
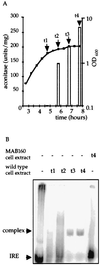
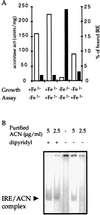
The aconitase protein of Bacillus subtilis was able to bind specifically to sequences resembling the iron response elements (IREs) found in eukaryotic mRNAs. The sequences bound include the rabbit ferritin IRE and IRE-like sequences in the B. subtilis operons that encode the major cytochrome oxidase and an iron uptake system. IRE binding activity was affected by the availability of iron both in vivo and in vitro. In eukaryotic cells, aconitase-like proteins regulate translation and stability of iron metabolism mRNAs in response to iron availability. A mutant strain of B. subtilis that produces an enzymatically inactive aconitase that was still able to bind RNA sporulated 40x more efficiently than did an aconitase null mutant, suggesting that a nonenzymatic activity of aconitase is important for sporulation. The results support the idea that bacterial aconitases, like their eukaryotic homologs, are bifunctional proteins, showing aconitase activity in the presence of iron and RNA binding activity when cells are iron-deprived.
Figures

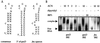
References
-
- Rouault T A, Klausner R D. Trends Biochem Sci. 1996;21:174–178. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

