Tracking neuronal fiber pathways in the living human brain
- PMID: 10468624
- PMCID: PMC17904
- DOI: 10.1073/pnas.96.18.10422
Tracking neuronal fiber pathways in the living human brain
Abstract
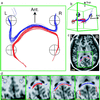
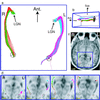
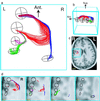
Functional imaging with positron emission tomography and functional MRI has revolutionized studies of the human brain. Understanding the organization of brain systems, especially those used for cognition, remains limited, however, because no methods currently exist for noninvasive tracking of neuronal connections between functional regions [Crick, F. & Jones, E. (1993) Nature (London) 361, 109-110]. Detailed connectivities have been studied in animals through invasive tracer techniques, but these invasive studies cannot be done in humans, and animal results cannot always be extrapolated to human systems. We have developed noninvasive neuronal fiber tracking for use in living humans, utilizing the unique ability of MRI to characterize water diffusion. We reconstructed fiber trajectories throughout the brain by tracking the direction of fastest diffusion (the fiber direction) from a grid of seed points, and then selected tracks that join anatomically or functionally (functional MRI) defined regions. We demonstrate diffusion tracking of fiber bundles in a variety of white matter classes with examples in the corpus callosum, geniculo-calcarine, and subcortical association pathways. Tracks covered long distances, navigated through divergences and tight curves, and manifested topological separations in the geniculo-calcarine tract consistent with tracer studies in animals and retinotopy studies in humans. Additionally, previously undescribed topologies were revealed in the other pathways. This approach enhances the power of modern imaging by enabling study of fiber connections among anatomically and functionally defined brain regions in individual human subjects.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

