Colony-stimulating factor-1 (CSF-1) expression in the uteroplacental unit of mice with spontaneous and induced pregnancy loss
- PMID: 10469060
- PMCID: PMC1905371
- DOI: 10.1046/j.1365-2249.1999.00986.x
Colony-stimulating factor-1 (CSF-1) expression in the uteroplacental unit of mice with spontaneous and induced pregnancy loss
Abstract
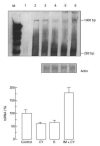
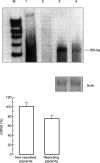
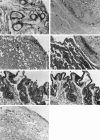
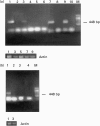
CSF-1 plays an important role in female reproduction and normal embryo development. To understand further CSF-1 function in normal and, especially, in compromised pregnancy, we studied the pattern of its mRNA expression as well as expression of its receptor (c-fms) in the uteroplacental units of mice with induced (cyclophosphamide (CY)-treated) and spontaneous (CBA/J x DBA/2J mating combination) pregnancy loss. RNase protection analysis demonstrated the presence of two forms of CSF-1 mRNA in the uteroplacental unit corresponding to 1400- and 263-bp protective fragments. Densitometric analysis demonstrated that the level of 1400-bp mRNA form was decreased by 40% in the uteroplacental units of mice with CY-induced pregnancy loss compared with the control mice. About 20% decrease in 263-bp protective fragment was registered in resorbing versus non-resorbed placenta of CBA/J females mated to DBA/2J males. As judged by in situ hybridization assay, CSF-1 mRNA transcripts were localized in the uterine epithelium and stroma, while c-fms mRNA was found mainly in the trophoblast. The number of metrial gland cells as well as the number of uterine leucocytes expressing CSF-1 and c-fms mRNAs was substantially lower in the uteroplacental unit of mice with pregnancy loss than in control animals. Maternal immunostimulation, while significantly decreasing the resorption rate in mice with CY-induced pregnancy loss, also strengthened CSF-1 mRNA expression at the fetomaternal interface and resulted in reconstitution in the number of CSF-1+ uterine leucocytes and metrial gland cells. These data suggest a role for uterine CSF-1 in the physiology of normal and compromised pregnancy and demonstrate a possible involvement of CSF-1-associated signalling in mechanisms of placenta and endometrium repair following immunopotentiation.
Figures
References
-
- Wegmann TG. The cytokine basis for cross-talk between the maternal immune and reproductive systems. Curr Opin Immunol. 1990;2:765–9. - PubMed
-
- Toder V, Shomer B. The role of lymphokines in pregnancy. Immunol Allerg Clin N Am. 1990;10:65–69.
-
- Stanley ER, Guilbert LJ, Tushinsky RJ, Bartelmez SH. CSF-1—a mononuclear phagocyte lineage-specific hemopoietic growth factor. J Cell Biochem. 1983;21:151–9. - PubMed
-
- De M, Sanford T, Wood GW. Relationship between macrophage colony-stimulating factor production by uterine epithelial cells and accumulation and distribution of macrophages in the uterus of pregnant mice. J Leukoc Biol. 1993;53:240–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

