Cyclic nucleotide-gated channels. Pore topology studied through the accessibility of reporter cysteines
- PMID: 10469728
- PMCID: PMC2229457
- DOI: 10.1085/jgp.114.3.377
Cyclic nucleotide-gated channels. Pore topology studied through the accessibility of reporter cysteines
Abstract
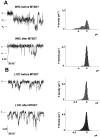
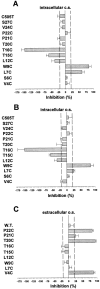
In voltage- and cyclic nucleotide-gated ion channels, the amino-acid loop that connects the S5 and S6 transmembrane domains, is a major component of the channel pore. It determines ion selectivity and participates in gating. In the alpha subunit of cyclic nucleotide-gated channels from bovine rod, the pore loop is formed by the residues R345-S371, here called R1-S27. These 24 residues were mutated one by one into a cysteine. Mutant channels were expressed in Xenopus laevis oocytes and currents were recorded from excised membrane patches. The accessibility of the substituted cysteines from both sides of the plasma membrane was tested with the thiol-specific reagents 2-aminoethyl methanethiosulfonate (MTSEA) and [2-(trimethylammonium)ethyl]methanethiosulfonate (MTSET). Residues V4C, T20C, and P22C were accessible to MTSET only from the external side of the plasma membrane, and to MTSEA from both sides of the plasma membrane. The effect of MTSEA applied to the inner side of T20C and P22C was prevented by adding 10 mM cysteine to the external side of the plasma membrane. W9C was accessible to MTSET from the internal side only. L7C residue was accessible to internal MTSET, but the inhibition was partial, approximately 50% when the MTS compound was applied in the absence of cGMP and 25% when it was applied in the presence of cGMP, suggesting that this residue is not located inside the pore lumen and that it changes its position during gating. Currents from T15C and T16C mutants were rapidly potentiated by intracellular MTSET. In T16C, a slower partial inhibition took place after the initial potentiation. Current from I17C progressively decayed in inside-out patches. The rundown was accelerated by inwardly applied MTSET. The accessibility results of MTSET indicate a well-defined topology of the channel pore in which residues between L7 and I17 are inwardly accessible, residue G18 and E19 form the narrowest section of the pore, and T20, P21, P22 and V4 are outwardly accessible.
Figures









References
-
- Akabas M.H., Stauffer D.A., Xu M., Karlin A. Acetylcholine receptor channel structure probed in cysteine-substitution mutants. Science. 1992;258:307–310. - PubMed
-
- Akabas M.H., Kauffmann C., Archdeacon P., Karlin A. Identification of acetylcholine receptor channel-lining residues in the entire M2 segment of the alpha subunit. Neuron. 1994;13:919–927. - PubMed
-
- Baylor D., Yau K.-W. Cyclic GMP-activated conductance of retinal photoreceptor cells. Annu. Rev. Neurosci. 1989;12:289–327. - PubMed
-
- Becchetti A., Gamel K. The properties of cysteine mutants in the pore region of cyclic nucleotide-gated channels. Pflügers Arch. 1999;In press - PubMed

