Histatin 3-mediated killing of Candida albicans: effect of extracellular salt concentration on binding and internalization
- PMID: 10471575
- PMCID: PMC89457
- DOI: 10.1128/AAC.43.9.2256
Histatin 3-mediated killing of Candida albicans: effect of extracellular salt concentration on binding and internalization
Abstract
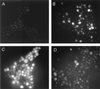
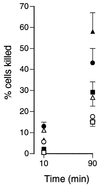
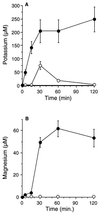
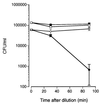
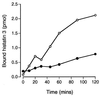
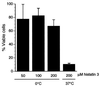
Human saliva contains histidine-rich proteins, histatins, which have antifungal activity in vitro. The mechanism by which histatins are able to kill Candida albicans may have clinical significance but is currently unknown. Using radiolabeled histatin 3, we show that the protein binds to C. albicans spheroplasts in a manner that is dependent on time and concentration. Binding to the spheroplasts was saturable and could be competed with unlabeled histatin 3. A single histatin 3 binding site with a K(d) = 5.1 microM was detected. Histatin 3 binding resulted in potassium and magnesium efflux, predominantly within the first 30 min of incubation. Studies with fluorescent histatin 3 demonstrate that the protein is internalized by C. albicans and that translocation of histatin inside the cell is closely associated with cell death. Histatin binding, internalization, and cell death are accelerated in low-ionic-strength conditions. Indeed, a low extracellular salt concentration was essential for cell death to occur, even when histatin 3 was already bound to the cell. The interaction of histatin 3 with C. albicans, and subsequent cell death, is inhibited at low temperature. These results demonstrate that the candidacidal activity of histatin 3 is not due exclusively to binding at the cell surface but also involves subsequent interactions with the cell.
Figures

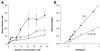

References
-
- Azen E A, Leutenegger W, Peters E H. Evolutionary and dietary aspects of salivary basic (Pb) and post Pb (PPb) proteins in anthropod primates. Nature. 1978;273:775–778. - PubMed
-
- Balekjian A Y, Longton R W. Histones isolated from human parotid fluid. Biochem Biophys Res Commun. 1973;50:676–682. - PubMed
-
- Brant E C, Santarpia R P, Pollock J J. Role of pH in salivary histidine-rich polypeptide antifungal germ tube inhibitory activity. Oral Microbiol Immunol. 1990;5:336–339. - PubMed
-
- Dawes C. The effects of flow rate and duration of stimulation on the concentrations of protein and the main electrolytes in human submandibular saliva. Arch Oral Biol. 1974;19:887–895. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

