Identification of an ATP-driven, osmoregulated glycine betaine transport system in Listeria monocytogenes
- PMID: 10473414
- PMCID: PMC99739
- DOI: 10.1128/AEM.65.9.4040-4048.1999
Identification of an ATP-driven, osmoregulated glycine betaine transport system in Listeria monocytogenes
Abstract
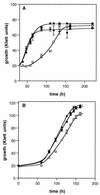
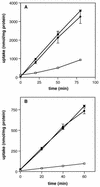
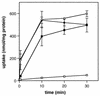
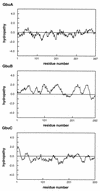
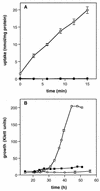
The ability of the gram-positive, food-borne pathogen Listeria monocytogenes to tolerate environments of elevated osmolarity and reduced temperature is due in part to the transport and accumulation of the osmolyte glycine betaine. Previously we showed that glycine betaine transport was the result of Na(+)-glycine betaine symport. In this report, we identify a second glycine betaine transporter from L. monocytogenes which is osmotically activated but does not require a high concentration of Na(+) for activity. By using a pool of Tn917-LTV3 mutants, a salt- and chill-sensitive mutant which was also found to be impaired in its ability to transport glycine betaine was isolated. DNA sequence analysis of the region flanking the site of transposon insertion revealed three open reading frames homologous to opuA from Bacillus subtilis and proU from Escherichia coli, both of which encode glycine betaine transport systems that belong to the superfamily of ATP-dependent transporters. The three open reading frames are closely spaced, suggesting that they are arranged in an operon. Moreover, a region upstream from the first reading frame was found to be homologous to the promoter regions of both opuA and proU. One unusual feature not shared with these other two systems is that the start codons for two of the open reading frames in L. monocytogenes appear to be TTG. That glycine betaine uptake is nearly eliminated in the mutant strain when it is assayed in the absence of Na(+) is an indication that only the ATP-dependent transporter and the Na(+)-glycine betaine symporter occur in L. monocytogenes.
Figures



Similar articles
-
Identification and disruption of BetL, a secondary glycine betaine transport system linked to the salt tolerance of Listeria monocytogenes LO28.Appl Environ Microbiol. 1999 May;65(5):2078-83. doi: 10.1128/AEM.65.5.2078-2083.1999. Appl Environ Microbiol. 1999. PMID: 10224004 Free PMC article.
-
Three transport systems for the osmoprotectant glycine betaine operate in Bacillus subtilis: characterization of OpuD.J Bacteriol. 1996 Sep;178(17):5071-9. doi: 10.1128/jb.178.17.5071-5079.1996. J Bacteriol. 1996. PMID: 8752321 Free PMC article.
-
OpuA, an osmotically regulated binding protein-dependent transport system for the osmoprotectant glycine betaine in Bacillus subtilis.J Biol Chem. 1995 Jul 14;270(28):16701-13. doi: 10.1074/jbc.270.28.16701. J Biol Chem. 1995. PMID: 7622480
-
Characterization of OpuA, a glycine-betaine uptake system of Lactococcus lactis.J Mol Microbiol Biotechnol. 2000 Apr;2(2):199-205. J Mol Microbiol Biotechnol. 2000. PMID: 10939245
-
Adaptation of Escherichia coli to high osmolarity environments: osmoregulation of the high-affinity glycine betaine transport system proU.FEMS Microbiol Rev. 1994 May;14(1):3-20. doi: 10.1111/j.1574-6976.1994.tb00067.x. FEMS Microbiol Rev. 1994. PMID: 8011357 Review.
Cited by
-
A single point mutation in the listerial betL σ(A)-dependent promoter leads to improved osmo- and chill-tolerance and a morphological shift at elevated osmolarity.Bioengineered. 2013 Nov-Dec;4(6):401-7. doi: 10.4161/bioe.24094. Epub 2013 Mar 11. Bioengineered. 2013. PMID: 23478432 Free PMC article.
-
Regulation of transcription of compatible solute transporters by the general stress sigma factor, sigmaB, in Listeria monocytogenes.J Bacteriol. 2004 Feb;186(3):794-802. doi: 10.1128/JB.186.3.794-802.2004. J Bacteriol. 2004. PMID: 14729706 Free PMC article.
-
Characterization of glycine betaine porter I from Listeria monocytogenes and its roles in salt and chill tolerance.Appl Environ Microbiol. 2002 Feb;68(2):813-9. doi: 10.1128/AEM.68.2.813-819.2002. Appl Environ Microbiol. 2002. PMID: 11823223 Free PMC article.
-
Multi-Target Antibacterial Mechanism of Moringin From Moringa oleifera Seeds Against Listeria monocytogenes.Front Microbiol. 2022 Jun 8;13:925291. doi: 10.3389/fmicb.2022.925291. eCollection 2022. Front Microbiol. 2022. PMID: 35756047 Free PMC article.
-
Genome-Based Characterization of Biological Processes That Differentiate Closely Related Bacteria.Front Microbiol. 2018 Feb 6;9:113. doi: 10.3389/fmicb.2018.00113. eCollection 2018. Front Microbiol. 2018. PMID: 29467735 Free PMC article.
References
-
- Ames G F-L. Structure and mechanism of bacterial periplasmic transport systems. J Bioenerg Biomembr. 1988;20:1–18. - PubMed
-
- Cole M B, Jones M V, Holyoak C. The effect of pH, salt concentration and temperature on the survival and growth of Listeria monocytogenes. J Appl Bacteriol. 1990;69:63–72. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

