A novel Ras-interacting protein required for chemotaxis and cyclic adenosine monophosphate signal relay in Dictyostelium
- PMID: 10473630
- PMCID: PMC25521
- DOI: 10.1091/mbc.10.9.2829
A novel Ras-interacting protein required for chemotaxis and cyclic adenosine monophosphate signal relay in Dictyostelium
Abstract
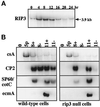
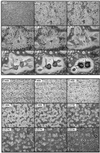
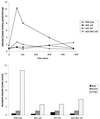
We have identified a novel Ras-interacting protein from Dictyostelium, RIP3, whose function is required for both chemotaxis and the synthesis and relay of the cyclic AMP (cAMP) chemoattractant signal. rip3 null cells are unable to aggregate and lack receptor activation of adenylyl cyclase but are able, in response to cAMP, to induce aggregation-stage, postaggregative, and cell-type-specific gene expression in suspension culture. In addition, rip3 null cells are unable to properly polarize in a cAMP gradient and chemotaxis is highly impaired. We demonstrate that cAMP stimulation of guanylyl cyclase, which is required for chemotaxis, is reduced approximately 60% in rip3 null cells. This reduced activation of guanylyl cyclase may account, in part, for the defect in chemotaxis. When cells are pulsed with cAMP for 5 h to mimic the endogenous cAMP oscillations that occur in wild-type strains, the cells will form aggregates, most of which, however, arrest at the mound stage. Unlike the response seen in wild-type strains, the rip3 null cell aggregates that form under these experimental conditions are very small, which is probably due to the rip3 null cell chemotaxis defect. Many of the phenotypes of the rip3 null cell, including the inability to activate adenylyl cyclase in response to cAMP and defects in chemotaxis, are very similar to those of strains carrying a disruption of the gene encoding the putative Ras exchange factor AleA. We demonstrate that aleA null cells also exhibit a defect in cAMP-mediated activation of guanylyl cyclase similar to that of rip3 null cells. A double-knockout mutant (rip3/aleA null cells) exhibits a further reduction in receptor activation of guanylyl cyclase, and these cells display almost no cell polarization or movement in cAMP gradients. As RIP3 preferentially interacts with an activated form of the Dictyostelium Ras protein RasG, which itself is important for cell movement, we propose that RIP3 and AleA are components of a Ras-regulated pathway involved in integrating chemotaxis and signal relay pathways that are essential for aggregation.
Figures









References
-
- Aubry L, Maeda M, Insall R, Devreotes PN, Firtel RA. The Dictyostelium mitogen-activated protein kinase ERK2 is regulated by Ras and cAMP-dependent protein kinase (PKA) and mediates PKA function. J Biol Chem. 1997;272:3883–3886. - PubMed
-
- Burki E, Anjard C, Scholder JC, Reymond CD. Isolation of two genes encoding putative protein kinases regulated during Dictyostelium discoideum development. Gene. 1991;102:57–65. - PubMed
-
- Chen MY, Insall RH, Devreotes PN. Signaling through chemoattractant receptors in Dictyostelium. Trends Genet. 1996;12:52–57. - PubMed
-
- Chung CY, Firtel RA. Dictyostelium: a model experimental system for elucidating the pathways and mechanisms controlling chemotaxis. In: Conn PM, Means A, editors. Molecular Regulation. Totowa, NJ: Humana Press; 1999. (in press).
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

