The B-oligomer of pertussis toxin deactivates CC chemokine receptor 5 and blocks entry of M-tropic HIV-1 strains
- PMID: 10477545
- PMCID: PMC2195621
- DOI: 10.1084/jem.190.5.597
The B-oligomer of pertussis toxin deactivates CC chemokine receptor 5 and blocks entry of M-tropic HIV-1 strains
Abstract
Infection of target cells by HIV-1 requires initial binding interactions between the viral envelope glycoprotein gp120, the cell surface protein CD4, and one of the members of the seven-transmembrane G protein-coupled chemokine receptor family. Most primary isolates (R5 strains) use chemokine receptor CCR5, but some primary syncytium-inducing, as well as T cell line-adapted, strains (X4 strains) use the CXCR4 receptor. Signaling from both CCR5 and CXCR4 is mediated by pertussis toxin (PTX)-sensitive G(i) proteins and is not required for HIV-1 entry. Here, we show that the PTX holotoxin as well as its binding subunit, B-oligomer, which lacks G(i)-inhibitory activity, blocked entry of R5 but not X4 strains into primary T lymphocytes. Interestingly, B-oligomer inhibited virus production by peripheral blood mononuclear cell cultures infected with either R5 or X4 strains, indicating that it can affect HIV-1 replication at both entry and post-entry levels. T cells treated with B-oligomer did not initiate signal transduction in response to macrophage inflammatory protein (MIP)-1beta or RANTES (regulated upon activation, normal T cell expressed and secreted); however, cell surface expression of CCR5 and binding of MIP-1beta or HIV-1 to such cells were not impaired. The inhibitory effect of B-oligomer on signaling from CCR5 and on entry of R5 HIV-1 strains was reversed by protein kinase C (PKC) inhibitors, indicating that B-oligomer activity is mediated by signaling events that involve PKC. B-oligomer also blocked cocapping of CCR5 and CD4 induced by R5 HIV-1 in primary T cells, but did not affect cocapping of CXCR4 and CD4 after inoculation of the cultures with X4 HIV-1. These results suggest that the B-oligomer of PTX cross-deactivates CCR5 to impair its function as a coreceptor for HIV-1.
Figures
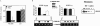




Comment in
-
Interference with the signaling capacity of CC chemokine receptor 5 can compromise its role as an HIV-1 entry coreceptor in primary T lymphocytes.J Exp Med. 1999 Sep 6;190(5):591-5. doi: 10.1084/jem.190.5.591. J Exp Med. 1999. PMID: 10477544 Free PMC article. Review. No abstract available.
References
-
- Maddon P.J., Dalgleish A.G., McDougal J.S., Clapham P.R., Weiss R.A., Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986;47:333–348. - PubMed
-
- Bieniasz P.D., Cullen B.R. Chemokine receptors and human immunodeficiency virus infection. Front. Biosci. (Online). 1998;3:D44–D58. - PubMed
-
- Alkhatib G., Combadiere C., Broder C.C., Feng Y., Kennedy P.E., Murphy P.M., Berger E.A. CC CKR5a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. - PubMed
-
- Choe H., Farzan M., Sun Y., Sullivan N., Rollins B., Ponath P.D., Wu L., Mackay C.R., LaRosa G., Newman W. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. - PubMed
-
- Deng H., Liu R., Ellmeier W., Choe S., Unutmaz D., Burkhart M., Di Marzio P., Marmon S., Sutton R.E., Hill C.M. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

