Megabase chromatin domains involved in DNA double-strand breaks in vivo
- PMID: 10477747
- PMCID: PMC2169482
- DOI: 10.1083/jcb.146.5.905
Megabase chromatin domains involved in DNA double-strand breaks in vivo
Abstract
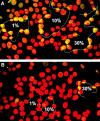
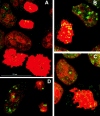
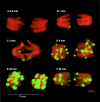
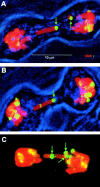
The loss of chromosomal integrity from DNA double-strand breaks introduced into mammalian cells by ionizing radiation results in the specific phosphorylation of histone H2AX on serine residue 139, yielding a specific modified form named gamma-H2AX. An antibody prepared to the unique region of human gamma-H2AX shows that H2AX homologues are phosphorylated not only in irradiated mammalian cells but also in irradiated cells from other species, including Xenopus laevis, Drosophila melanogaster, and Saccharomyces cerevisiae. The antibody reveals that gamma-H2AX appears as discrete nuclear foci within 1 min after exposure of cells to ionizing radiation. The numbers of these foci are comparable to the numbers of induced DNA double-strand breaks. When DNA double-strand breaks are introduced into specific partial nuclear volumes of cells by means of a pulsed microbeam laser, gamma-H2AX foci form at these sites. In mitotic cells from cultures exposed to nonlethal amounts of ionizing radiation, gamma-H2AX foci form band-like structures on chromosome arms and on the end of broken arms. These results offer direct visual confirmation that gamma-H2AX forms en masse at chromosomal sites of DNA double-strand breaks. The results further suggest the possible existence of units of higher order chromatin structure involved in monitoring DNA integrity.
Figures


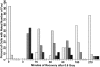
References
-
- Anderson C.W. Protein kinases and the response to DNA damage. Semin. Cell Biol. 1994;5:427–436. - PubMed
-
- Bai Y., Symington L.S. A RAD52 homolog is required for RAD51-independent mitotic recombination in Saccharomyces cerevisiae . Genes Dev. 1996;10:2025–2037. - PubMed
-
- Cedervall B., Wong R., Albright N., Dynlacht J., Lambin P., Dewey W.C. Methods for the quantification of DNA double-strand breaks determined from the distribution of DNA fragment sizes measured by pulsed-field gel electrophoresis. Radiat. Res. 1995;143:8–16. - PubMed
-
- Celis J.E. Cell Biology. Vol. 2. 2nd ed Academic Press 1998. 533 San Diego; CA: pp
-
- Doida Y., Okada S. Radiation-induced mitotic delay in cultured mammalian cells (L5178Y) Radiat. Res. 1969;38:513–529. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

