Microtubule targeting of substrate contacts promotes their relaxation and dissociation
- PMID: 10477757
- PMCID: PMC2169483
- DOI: 10.1083/jcb.146.5.1033
Microtubule targeting of substrate contacts promotes their relaxation and dissociation
Abstract
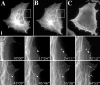
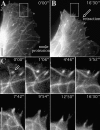
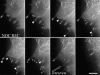
We recently showed that substrate contact sites in living fibroblasts are specifically targeted by microtubules (Kaverina, I., K. Rottner, and J.V. Small. 1998. J. Cell Biol. 142:181-190). Evidence is now provided that microtubule contact targeting plays a role in the modulation of substrate contact dynamics. The results are derived from spreading and polarized goldfish fibroblasts in which microtubules and contact sites were simultaneously visualized using proteins conjugated with Cy-3, rhodamine, or green fluorescent protein. For cells allowed to spread in the presence of nocodazole the turnover of contacts was retarded, as compared with controls and adhesions that were retained under the cell body were dissociated after microtubule reassembly. In polarized cells, small focal complexes were found at the protruding cell front and larger adhesions, corresponding to focal adhesions, at the retracting flanks and rear. At retracting edges, multiple microtubule contact targeting preceded contact release and cell edge retraction. The same effect could be observed in spread cells, in which microtubules were allowed to reassemble after local disassembly by the application of nocodazole to one cell edge. At the protruding front of polarized cells, focal complexes were also targeted and as a result remained either unchanged in size or, more rarely, were disassembled. Conversely, when contact targeting at the cell front was prevented by freezing microtubule growth with 20 nM taxol and protrusion stimulated by the injection of constitutively active Rac, peripheral focal complexes became abnormally enlarged. We further found that the local application of inhibitors of myosin contractility to cell edges bearing focal adhesions induced the same contact dissociation and edge retraction as observed after microtubule targeting. Our data are consistent with a mechanism whereby microtubules deliver localized doses of relaxing signals to contact sites to retard or reverse their development. We propose that it is via this route that microtubules exert their well-established control on cell polarity.
Figures








References
-
- Bershadsky A.D., Vasiliev J.M. Cytoskeleton 1988. Plenum Press; New York: pp. 298 pp
-
- Bershadsky A., Chausovsky A., Becker E., Lyubimova A., Geiger B. Involvement of microtubules in the control of adhesion-dependent signal transduction. Curr. Biol. 1996;6:1279–1289. - PubMed
-
- Best A., Ahmed S., Kozma R., Lim L. The Ras-related GTPase Rac1 binds tubulin. J. Biol. Chem. 1996;271:3756–3762. - PubMed
-
- Chen W.-T. Surface changes during retraction-induced spreading of fibroblasts. J. Cell Sci. 1981;49:1–13. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

