Myosin Va movements in normal and dilute-lethal axons provide support for a dual filament motor complex
- PMID: 10477758
- PMCID: PMC2169472
- DOI: 10.1083/jcb.146.5.1045
Myosin Va movements in normal and dilute-lethal axons provide support for a dual filament motor complex
Abstract
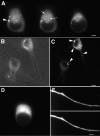
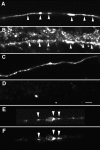
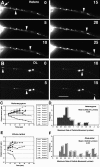
To investigate the role that myosin Va plays in axonal transport of organelles, myosin Va-associated organelle movements were monitored in living neurons using microinjected fluorescently labeled antibodies to myosin Va or expression of a green fluorescent protein-myosin Va tail construct. Myosin Va-associated organelles made rapid bi-directional movements in both normal and dilute-lethal (myosin Va null) neurites. In normal neurons, depolymerization of microtubules by nocodazole slowed, but did not stop movement. In contrast, depolymerization of microtubules in dilute-lethal neurons stopped movement. Myosin Va or synaptic vesicle protein 2 (SV2), which partially colocalizes with myosin Va on organelles, did not accumulate in dilute-lethal neuronal cell bodies because of an anterograde bias associated with organelle transport. However, SV2 showed peripheral accumulations in axon regions of dilute-lethal neurons rich in tyrosinated tubulin. This suggests that myosin Va-associated organelles become stranded in regions rich in dynamic microtubule endings. Consistent with these observations, presynaptic terminals of cerebellar granule cells in dilute-lethal mice showed increased cross-sectional area, and had greater numbers of both synaptic and larger SV2 positive vesicles. Together, these results indicate that myosin Va binds to organelles that are transported in axons along microtubules. This is consistent with both actin- and microtubule-based motors being present on these organelles. Although myosin V activity is not necessary for long-range transport in axons, myosin Va activity is necessary for local movement or processing of organelles in regions, such as presynaptic terminals that lack microtubules.
Figures








References
-
- Bizario J.C.S., Rosa L.C., Nascimento A.A.C., Larson R.E., Espreafico E.M. Melanosome distribution is affected by expression of chicken myosin-Va constructs in wild type and dilute viral melanoma cells Mol. Biol. Cell. 9 1998. 20a(Abstr.)
-
- Cheney R.E., O'Shea M.K., Heuser J.E., Coelho M.V., Wolenski J.S., Espreafico E.M., Forscher P., Larson R.E., Mooseker M.S. Brain myosin-V is a two-headed unconventional myosin with motor activity. Cell. 1993;75:13–23. - PubMed

