Regulation of cell contraction and membrane ruffling by distinct signals in migratory cells
- PMID: 10477763
- PMCID: PMC2169492
- DOI: 10.1083/jcb.146.5.1107
Regulation of cell contraction and membrane ruffling by distinct signals in migratory cells
Abstract
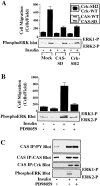
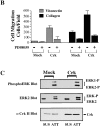
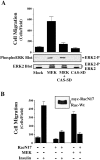
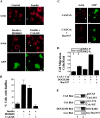
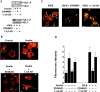
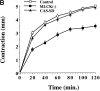
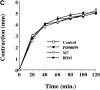
Cell migration and wound contraction requires assembly of actin into a functional myosin motor unit capable of generating force. However, cell migration also involves formation of actin-containing membrane ruffles. Evidence is provided that actin-myosin assembly and membrane ruffling are regulated by distinct signaling pathways in the migratory cell. Interaction of cells with extracellular matrix proteins or cytokines promote cell migration through activation of the MAP kinases ERK1 and ERK2 as well as the molecular coupling of the adaptor proteins p130CAS and c-CrkII. ERK signaling is independent of CAS/Crk coupling and regulates myosin light chain phosphorylation leading to actin-myosin assembly during cell migration and cell-mediated contraction of a collagen matrix. In contrast, membrane ruffling, but not cell contraction, requires Rac GTPase activity and the formation of a CAS/Crk complex that functions in the context of the Rac activating protein DOCK180. Thus, during cell migration ERK and CAS/Crk coupling operate as components of distinct signaling pathways that control actin assembly into myosin motors and membrane ruffles, respectively.
Figures


References
-
- Altun-Glutekin Z.F., Chandrian S., Bougert C., Ishizaki I., Narumiya S., de Graaf P., Van Bergen en Henegouwen P., Hanafusa H., Wagner J.A., Birge R.B. Activation of Rho-dependent cell spreading and focal adhesion biogenesis by the v-Crk adaptor protein. Mol. Cell Biol. 1998;18:3044–3058. - PMC - PubMed
-
- Anand-Apte B., Zetter B.R., Viswanathan A., Qui R., Chen J., Ruggeri R., Symons M. Platelet-derived growth factor and fibronectin-stimulated migration are differentially regulated by the Rac and extracellular signal-regulated kinase pathways. J. Biol. Chem. 1997;272:30688–30692. - PubMed
-
- Aplin A.E., Howe A., Alahari S.K., Juliano R.L. Signal transduction and signal modulation by cell adhesion receptorsthe role of integrins, cadherins immunoglobulin-cell adhesion molecules, and selectins. Pharm. Rev. 1998;50:197–263. - PubMed
-
- Blaukat A., Ivankovic-Dikic I., Gronroos E., Dolfi F., Tokiwa G., Vuori K., Dikic I. Adaptor proteins Grb2 and Crk couple Pyk2 with activation of specific mitogen-activated kinase cascades. J. Biol. Chem. 1999;274:14893–14901. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

