Ultrastructural localization of full-length trkB immunoreactivity in rat hippocampus suggests multiple roles in modulating activity-dependent synaptic plasticity
- PMID: 10479701
- PMCID: PMC6782460
- DOI: 10.1523/JNEUROSCI.19-18-08009.1999
Ultrastructural localization of full-length trkB immunoreactivity in rat hippocampus suggests multiple roles in modulating activity-dependent synaptic plasticity
Abstract
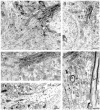
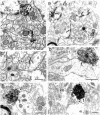
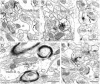
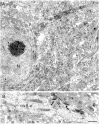
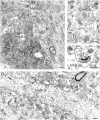
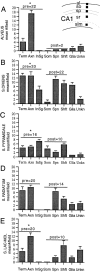
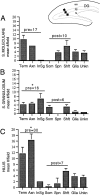
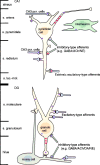
Neurotrophins acting at the trkB receptor have been shown to be important modulators of activity-dependent plasticity in the hippocampus, but the mechanisms underlying these effects are not yet well understood. To identify the cellular and subcellular targets of trkB ligands in the adult rat hippocampal formation, full-length trkB receptor immunoreactivity (trkB-IR) was localized using electron microscopy. trkB-IR was present in the glutamatergic pyramidal and granule cells. Labeling in these neurons appeared as discrete clusters and was primarily in axons, excitatory-type axon terminals, and dendritic spines and to a lesser extent in somata and dendritic shafts. trkB-IR was commonly found on the plasma membrane of dendritic spines, whereas in other subcellular regions trkB-IR was often intracellular. Labeling was strikingly dense within axon initial segments, suggesting extensive receptor trafficking. trkB-IR was not confined to pyramidal and granule cells. Dense trkB-IR was found in occasional interneuron axon initial segments, some axon terminals forming inhibitory-type synapses onto somata and dendritic shafts, and excitatory-type terminals likely to originate extrahippocampally. This suggests that trkB is contained in some GABAergic interneurons, neuromodulatory (e.g., cholinergic, dopaminergic, and noradrenergic) afferents, and/or glutamatergic afferents. These data indicate that full-length trkB receptor activation may modulate glutamatergic pathways of the trisynaptic circuit both presynaptically at axon terminals and initial segments and postsynaptically at dendritic spines and shafts. Signaling via catalytic trkB may also presynaptically affect inhibitory and modulatory neurons. A pan-trkB antibody labeled the same neuronal populations as the full-length-specific trkB antiserum, but the labels differed in density at various subcellular sites. These findings provide an ultrastructural foundation for further examining the mechanisms through which neurotrophins acting at trkB receptors contribute to synaptic plasticity.
Figures



References
-
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. - PubMed
-
- Alderson RF, Alterman AL, Barde YA, Lindsay RM. Brain-derived neurotrophic factor increases survival and differentiated functions of rat septal cholinergic neurons in culture. Neuron. 1990;5:297–306. - PubMed
-
- Alkondon M, Pereira EF, Barbosa CT, Albuquerque EX. Neuronal nicotinic acetylcholine receptor activation modulates gamma-aminobutyric acid release from CA1 neurons of rat hippocampal slices. J Pharmacol Exp Ther. 1997;283:1396–1411. - PubMed
-
- Altar CA, DiStefano PS. Neurotrophin trafficking by anterograde transport. Trends Neurosci. 1998;21:433–437. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
