Neuronal interactions improve cortical population coding of movement direction
- PMID: 10479708
- PMCID: PMC6782478
- DOI: 10.1523/JNEUROSCI.19-18-08083.1999
Neuronal interactions improve cortical population coding of movement direction
Abstract
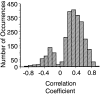
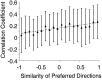
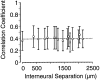
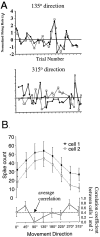
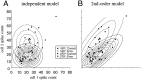
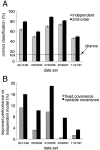
Interactions among groups of neurons in primary motor cortex (MI) may convey information about motor behavior. We investigated the information carried by interactions in MI of macaque monkeys using a novel multielectrode array to record simultaneously from 12-16 neurons during an arm-reaching task. Pairs of simultaneously recorded cells revealed significant correlations in their trial-to-trial firing rate variation when estimated over broad (600 msec) time intervals. This covariation was only weakly related to the preferred directions of the individual MI neurons estimated from the firing rate and did not vary significantly with interelectrode distance. Most significantly, in a portion of cell pairs, correlation strength varied with the direction of the arm movement. We evaluated to what extent correlated activity provided additional information about movement direction beyond that available in single neuron firing rate. A multivariate statistical model successfully classified direction from single trials of neural data. However, classification was consistently better when correlations were incorporated into the model as compared to one in which neurons were treated as independent encoders. Information-theoretic analysis demonstrated that interactions caused by correlated activity carry additional information about movement direction beyond that based on the firing rates of independently acting neurons. These results also show that cortical representations incorporating higher order features of population activity would be richer than codes based solely on firing rate, if such information can exploited by the nervous system.
Figures



References
-
- Abbott LF, Dayan P. The effect of correlated variability on the accuracy of a population code. Neural Comput. 1998;11:91–101. - PubMed
-
- Bair W, Koch C. Temporal precision of spike trains in extrastriate cortex of the behaving macaque monkey. Neural Comput. 1996;8:1185–1202. - PubMed
-
- Buracas GT, Zador AM, DeWeese MR, Albright TD. Efficient discrimination of temporal patterns by motion-sensitive neurons in primate visual cortex. Neuron. 1998;20:959–969. - PubMed
-
- Crow EL, Davis FA, Maxfield MW. Statistics Manual. Dover; New York: 1960.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
