Role of immune responses against the envelope and the core antigens of simian immunodeficiency virus SIVmne in protection against homologous cloned and uncloned virus challenge in Macaques
- PMID: 10482571
- PMCID: PMC112838
- DOI: 10.1128/JVI.73.10.8201-8215.1999
Role of immune responses against the envelope and the core antigens of simian immunodeficiency virus SIVmne in protection against homologous cloned and uncloned virus challenge in Macaques
Abstract
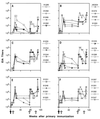
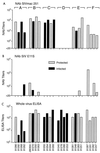
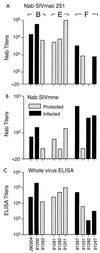
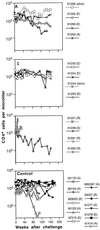
We previously showed that envelope (gp160)-based vaccines, used in a live recombinant virus priming and subunit protein boosting regimen, protected macaques against intravenous and intrarectal challenges with the homologous simian immunodeficiency virus SIVmne clone E11S. However, the breadth of protection appears to be limited, since the vaccines were only partially effective against intravenous challenge by the uncloned SIVmne. To examine factors that could affect the breadth and the efficacy of this immunization approach, we studied (i) the effect of priming by recombinant vaccinia virus; (ii) the role of surface antigen gp130; and (iii) the role of core antigens (Gag and Pol) in eliciting protective immunity. Results indicate that (i) priming with recombinant vaccinia virus was more effective than subunit antigen in eliciting protective responses; (ii) while both gp130 and gp160 elicited similar levels of SIV-specific antibodies, gp130 was not as effective as gp160 in protection, indicating a possible role for the transmembrane protein in presenting functionally important epitopes; and (iii) although animals immunized with core antigens failed to generate any neutralizing antibody and were infected upon challenge, their virus load was 50- to 100-fold lower than that of the controls, suggesting the importance of cellular immunity or other core-specific immune responses in controlling acute infection. Complete protection against intravenous infection by the pathogenic uncloned SIVmne was achieved by immunization with both the envelope and the core antigens. These results indicate that immune responses to both antigens may contribute to protection and thus argue for the inclusion of multiple antigens in recombinant vaccine designs.
Figures





Similar articles
-
Protection of macaques against intrarectal infection by a combination immunization regimen with recombinant simian immunodeficiency virus SIVmne gp160 vaccines.J Virol. 1999 Apr;73(4):3134-46. doi: 10.1128/JVI.73.4.3134-3146.1999. J Virol. 1999. PMID: 10074165 Free PMC article.
-
Limited breadth of the protective immunity elicited by simian immunodeficiency virus SIVmne gp160 vaccines in a combination immunization regimen.J Virol. 1999 Jan;73(1):618-30. doi: 10.1128/JVI.73.1.618-630.1999. J Virol. 1999. PMID: 9847367 Free PMC article.
-
Recombinant subunit vaccines as an approach to study correlates of protection against primate lentivirus infection.Immunol Lett. 1996 Jun;51(1-2):115-9. doi: 10.1016/0165-2478(96)02564-3. Immunol Lett. 1996. PMID: 8811354
-
Protection of vaccinia-primed macaques against SIVmne infection by combination immunization with recombinant vaccinia virus and SIVmne gp160.J Med Primatol. 1993 Feb-May;22(2-3):92-9. J Med Primatol. 1993. PMID: 8411113
-
Good CoP, bad CoP? Interrogating the immune responses to primate lentiviral vaccines.Retrovirology. 2012 Oct 1;9:80. doi: 10.1186/1742-4690-9-80. Retrovirology. 2012. PMID: 23025660 Free PMC article. Review.
Cited by
-
Oral Immunization with Recombinant Vaccinia Virus Prime and Intramuscular Protein Boost Provides Protection against Intrarectal Simian-Human Immunodeficiency Virus Challenge in Macaques.Clin Vaccine Immunol. 2015 Dec 30;23(3):204-12. doi: 10.1128/CVI.00597-15. Clin Vaccine Immunol. 2015. PMID: 26718849 Free PMC article.
-
DNA-MVA vaccine protection after X4 SHIV challenge in macaques correlates with day-of-challenge antiviral CD4+ cell-mediated immunity levels and postchallenge preservation of CD4+ T cell memory.AIDS Res Hum Retroviruses. 2008 Mar;24(3):505-19. doi: 10.1089/aid.2007.0191. AIDS Res Hum Retroviruses. 2008. PMID: 18373436 Free PMC article.
-
Utility of human immunodeficiency virus type 1 envelope as a T-cell immunogen.J Virol. 2007 Dec;81(23):13125-34. doi: 10.1128/JVI.01408-07. Epub 2007 Sep 26. J Virol. 2007. PMID: 17898063 Free PMC article.
-
Vaccine protection against simian immunodeficiency virus by recombinant strains of herpes simplex virus.J Virol. 2000 Sep;74(17):7745-54. doi: 10.1128/jvi.74.17.7745-7754.2000. J Virol. 2000. PMID: 10933680 Free PMC article.
-
Immunization with a modified vaccinia virus expressing simian immunodeficiency virus (SIV) Gag-Pol primes for an anamnestic Gag-specific cytotoxic T-lymphocyte response and is associated with reduction of viremia after SIV challenge.J Virol. 2000 Mar;74(6):2502-9. doi: 10.1128/jvi.74.6.2502-2509.2000. J Virol. 2000. PMID: 10684264 Free PMC article.
References
-
- Abimiku A G, Franchini G, Tartaglia J, Aldrich K, Myagkikh M, Markham P D, Chong P, Klein M, Kieny M P, Paoletti E, Gallo R C, Robert-Guroff M. HIV-1 recombinant poxvirus vaccine induces cross-protection against HIV-2 challenge in rhesus macaques. Nat Med. 1995;1:321–329. - PubMed
-
- Baba T W, Liska V, Kimani A H, Ray N B, Dailey P J, Penninck D, Bronson R, Greene M F, McClure H M, Martin L N, Ruprecht R M. Live attenuated, multiply deleted simian immunodeficiency virus causes AIDS in infant and adult macaques. Nat Med. 1999;5:194–203. - PubMed
-
- Benson J, Chougnet C, Robert-Guroff M, Montefiori D, Markham P, Shearer G, Gallo R C, Cranage M, Paoletti E, Limbach K, Venzon D, Tartaglia J, Franchini G. Recombinant vaccine-induced protection against highly pathogenic simian immunodeficiency virus SIVmac251 dependence on route of challenge exposure. J Virol. 1998;72:4170–4182. - PMC - PubMed
-
- Benveniste R E, Raben D, Hill R, Knott W, Drummond J E, Arthur L O, Jahrling P B, Morton W R, Henderson L E, Heidecker G. Molecular characterization and comparison of simian immunodeficiency virus isolates from macaques, mangabeys, and African green monkeys. J Med Primatol. 1989;18:287–303. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

