trans-dominant interference with human immunodeficiency virus type 1 replication and transmission in CD4(+) cells by an envelope double mutant
- PMID: 10482579
- PMCID: PMC112846
- DOI: 10.1128/JVI.73.10.8290-8302.1999
trans-dominant interference with human immunodeficiency virus type 1 replication and transmission in CD4(+) cells by an envelope double mutant
Abstract
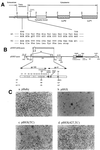
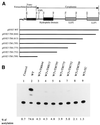
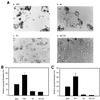
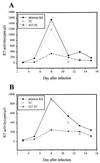
We previously reported that a human immunodeficiency virus type 1 (HIV-1) envelope (Env) mutant with the whole cytoplasmic domain deleted, denoted mutant TC, is able to dominantly interfere with wild-type (wt) virus infectivity. In the present study, the feasibility of developing a dominant negative mutant-based genetic anti-HIV strategy targeting the gp41 cytoplasmic domain was investigated. Mutants TC and 427,TC, a TC derivative with a Trp-to-Ser substitution introduced into residue 427 in the CD4-binding site, and a series of mutants with deletions in the cytoplasmic domain, effectively trans-dominantly interfered with wt Env-mediated viral infectivity, as demonstrated by an env trans-complementation assay. The syncytium formation-defective 427, TC double mutant not only inhibited heterologous LAV and ELI Env-mediated viral infectivity but also interfered with syncytium formation and infectivity mediated by the Env proteins of the two primary isolates 92BR and 92US. Stable HeLa-CD4-LTR-beta-gal clones that harbored Tat-controlled expression cassettes encoding the control DeltaKS, which had a deletion in the env gene, wt, or mutant env gene were generated. Viral transmission mediated by laboratory-adapted T-cell-tropic HXB2 and NL4-3 viruses was greatly reduced in the TC and 427,TC transfectants compared to that observed in the control DeltaKS and wt transfectants. Viral replication caused by HXB2 and NL4-3 viruses and by macrophage-tropic ConB and ADA-GG viruses was delayed or reduced in human CD4(+) T cells transfected with the 427,TC env construct compared to that observed in cells transfected with the control DeltaKS or TC env construct. The lack of significant interference by TC mutant was due neither to the lack of TC env gene integration into host DNA nor to the lack of TC Env expression upon Tat induction. These results indicate that this 427,TC Env double mutant has a role in the development of trans-dominant mutant-based genetic anti-HIV strategies.
Figures




References
-
- Abacioglu Y H, Fouts T R, Laman J D, Claasen E, Pincus S H, Moore J P, Roby C A, Kamin-Lewis R, Lewis G K. Epitope mapping and topology of baculovirus-expressed HIV-1 gp160 determined with a panel of murine monoclonal antibodies. AIDS Res Hum Retroviruses. 1994;10:371–381. - PubMed
-
- Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. Vol. 2. New York, N.Y: John Wiley & Sons; 1995.
-
- Buchschacher G L, Jr, Freed E O, Panganiban A T. Cells induced to express a human immunodeficiency virus type 1 envelope gene mutant inhibit the spread of wild-type virus. Hum Gene Ther. 1992;3:391–397. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

