Initial binding of murine leukemia virus particles to cells does not require specific Env-receptor interaction
- PMID: 10482613
- PMCID: PMC112880
- DOI: 10.1128/JVI.73.10.8599-8611.1999
Initial binding of murine leukemia virus particles to cells does not require specific Env-receptor interaction
Abstract
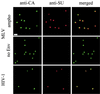
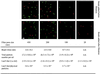
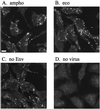
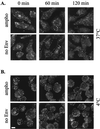
The initial step of virus-cell interaction was studied by immunofluorescence microscopy. Single particles of murine leukemia virus (MLV) vectors and human immunodeficiency virus (HIV) were visualized by immunofluorescence. Fluorescent dots representing single virions could be localized by staining of capsid proteins (CA) or surface envelope proteins (SU) after fixation of virus supernatants. This technique can be used to determine particle concentration in viral supernatants and also to study virus-cell interaction. We investigated the role of the Env-receptor interaction for the initial binding event between the cell and the viral particles. Ecotropic MLV vector particles were shown to bind to human cells which do not express the specific viral receptor. In addition, MLV particles defective for Env were shown to bind the cells similarly to infectious MLV. Time course experiments of virus-cell binding and dissociation showed identical profiles for infectious and Env-defective MLV particles and suggested that MLV Env is not involved in the early phases of attachment of virus to cells. The possible implication of cellular factors in enhancing viral binding and infectivity is discussed.
Figures




Similar articles
-
Multiple Gag domains contribute to selective recruitment of murine leukemia virus (MLV) Env to MLV virions.J Virol. 2013 Feb;87(3):1518-27. doi: 10.1128/JVI.02604-12. Epub 2012 Nov 14. J Virol. 2013. PMID: 23152533 Free PMC article.
-
IFITM3 Reduces Retroviral Envelope Abundance and Function and Is Counteracted by glycoGag.mBio. 2020 Jan 21;11(1):e03088-19. doi: 10.1128/mBio.03088-19. mBio. 2020. PMID: 31964738 Free PMC article.
-
Sequence Determinants in Gammaretroviral Env Cytoplasmic Tails Dictate Virus-Specific Pseudotyping Compatibility.J Virol. 2019 May 15;93(11):e02172-18. doi: 10.1128/JVI.02172-18. Print 2019 Jun 1. J Virol. 2019. PMID: 30894464 Free PMC article.
-
Characterization of producer cell-dependent restriction of murine leukemia virus replication.J Virol. 2002 Jul;76(13):6609-17. doi: 10.1128/jvi.76.13.6609-6617.2002. J Virol. 2002. PMID: 12050374 Free PMC article.
-
Xenotropism: the elusive viral receptor finally uncovered.Proc Natl Acad Sci U S A. 1999 Feb 2;96(3):802-4. doi: 10.1073/pnas.96.3.802. Proc Natl Acad Sci U S A. 1999. PMID: 9927648 Free PMC article. Review. No abstract available.
Cited by
-
Charged polymers modulate retrovirus transduction via membrane charge neutralization and virus aggregation.Biophys J. 2004 Feb;86(2):1234-42. doi: 10.1016/S0006-3495(04)74197-1. Biophys J. 2004. PMID: 14747357 Free PMC article.
-
Heparin binds to murine leukemia virus and inhibits Env-independent attachment and infection.J Virol. 2002 Jul;76(14):6909-18. doi: 10.1128/jvi.76.14.6909-6918.2002. J Virol. 2002. PMID: 12072492 Free PMC article.
-
Transmembrane domains of hepatitis C virus envelope glycoproteins: residues involved in E1E2 heterodimerization and involvement of these domains in virus entry.J Virol. 2007 Mar;81(5):2372-81. doi: 10.1128/JVI.02198-06. Epub 2006 Dec 13. J Virol. 2007. PMID: 17166909 Free PMC article.
-
DEAE-Dextran Enhances the Lentiviral Transduction of Primary Human Mesenchymal Stromal Cells from All Major Tissue Sources Without Affecting Their Proliferation and Phenotype.Mol Biotechnol. 2023 Apr;65(4):544-555. doi: 10.1007/s12033-022-00549-2. Epub 2022 Aug 23. Mol Biotechnol. 2023. PMID: 35999479 Free PMC article.
-
Dendritic cells and T cells deliver oncolytic reovirus for tumour killing despite pre-existing anti-viral immunity.Gene Ther. 2009 May;16(5):689-99. doi: 10.1038/gt.2009.29. Epub 2009 Mar 12. Gene Ther. 2009. PMID: 19282847 Free PMC article.
References
-
- Allison A C, Valentine R C. Virus particle adsorption. I. Theory of adsorption and experiments on the attachment of particles to non-biological surfaces. Biochim Biophys Acta. 1959;34:10–23. - PubMed
-
- Allison A C, Valentine R C. Virus particle adsorption. II. Adsorption of vaccinia and fowl plaque viruses to cells in suspension. Biochim Biophys Acta. 1959;40:393–399. - PubMed
-
- Allison A C, Valentine R C. Virus particle adsorption. III. Adsorption of viruses by cell monolayers and effect of some variables on adsorption. Biochim Biophys Acta. 1959;40:400–410. - PubMed
-
- Bartlett J S, Samulski R J. Fluorescent viral vectors: a new technique for the pharmacological analysis of gene therapy. Nat Med. 1998;4:635–637. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

