Carboxypeptidase D is an avian hepatitis B virus receptor
- PMID: 10482623
- PMCID: PMC112890
- DOI: 10.1128/JVI.73.10.8696-8702.1999
Carboxypeptidase D is an avian hepatitis B virus receptor
Abstract
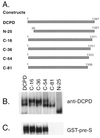
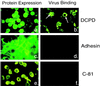
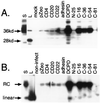
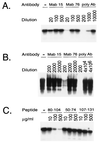
The receptor molecules for human and animal hepatitis B viruses have not been defined. Previous studies have described a 170 to 180 kDa molecule (p170 or gp180) that binds in vitro to the pre-S domain of the large envelope protein of duck hepatitis B virus (DHBV); cDNA cloning revealed the binding protein to be duck carboxypeptidase D (DCPD). In the present study, the DCPD cDNA was transfected into several nonpermissive human-, monkey-, and avian species-derived cell lines. Cells transfected with a plasmid encoding the full-length DCPD protein bound DHBV particles, whereas cells expressing truncated versions of DCPD protein that fail to bind the pre-S protein did not. The DHBV binding to DCPD-reconstituted cells was blocked by a monoclonal antibody that neutralizes DHBV infection of primary duck hepatocytes (PDH) and also by a pre-S peptide previously shown to inhibit DHBV infection of PDH. In addition to promoting virus binding, DCPD expression was associated with internalization of viral particles. The entry process was prevented by incubation of reconstituted cells with DHBV at 4 degrees C and by the addition of energy-depleting agents known to block DHBV entry into PDH. These results demonstrated that DCPD is a DHBV receptor. However, the lack of complete viral replication in DCPD-reconstituted cells suggested that additional factors are required for postentry events in immortalized cell lines.
Figures


References
-
- Beasley R P, Hwang L Y, Lin C C, Chien C S. Hepatocellular carcinoma and hepatitis B virus: a prospective study of 22702 men in Taiwan. Lancet. 1981;ii:1129–1133. - PubMed
-
- Chassot S, Lambert V, Kay A, Godinot A, Roux C, Trepo C, Cova L. Fine mapping of neutralization epitopes on duck hepatitis B virus (DHBV) pre-S protein using monoclonal antibodies and overlapping peptides. Virology. 1993;192:217–223. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

