Mechanically induced avoidance response of chloroplasts in fern protonemal cells
- PMID: 10482658
- PMCID: PMC59387
- DOI: 10.1104/pp.121.1.37
Mechanically induced avoidance response of chloroplasts in fern protonemal cells
Abstract
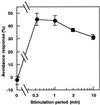
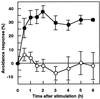
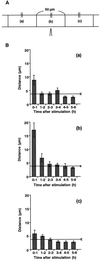
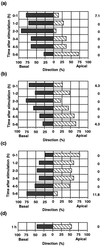
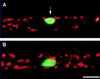
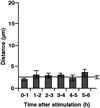
Cell response to mechanical stimulation was investigated at a subcellular level in protonemal cells of the fern Adiantum capillus-veneris L. by pressing a small part of the cell with a microcapillary. In cells receiving local stimulation, the chloroplasts moved away from the site of stimulation, whereas the nuclei failed to show such avoidance movement. Mechanical stimulation for a period as short as 0.3 min was enough to induce the avoidance response to a maximal level. The avoidance movement of chloroplasts started within 30 min and the plateau level of avoidance was attained around 2 h after stimulation. By tracing the movement of chloroplasts during the response, it was shown that the mobility of chloroplasts near the stimulation site increased transiently within 1 h after the stimulation. After 2 to 3 h, it slowed down to the control level without stimulation. The avoidance response was inhibited by 0.1 mM cytochalasin B and 25 mM 2, 3-butanedione monoxime but not by 3.3 microM amiprophosmethyl or 5 mM colchicine. These findings indicate that the protonemal cells were very sensitive to mechanical stimulation and that chloroplasts moved away from the mechanically stimulated site through the actomyosin motile system.
Figures




References
-
- Darwin C (1882) The Power of Movement in Plants. John Murray, London
-
- Grabski S, Arnoys E, Busch B, Schindler M. Regulation of actin tension in plant cells by kinases and phosphatases. Plant Physiol. 1998;116:279–290.
-
- Higuchi H, Takemori S. Butanedione monoxime suppresses contraction and ATPase activity of rabbit skeletal muscle. J Biochem. 1989;105:638–643. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

