Biochemical characterization of the suberization-associated anionic peroxidase of potato
- PMID: 10482668
- PMCID: PMC59361
- DOI: 10.1104/pp.121.1.135
Biochemical characterization of the suberization-associated anionic peroxidase of potato
Abstract
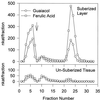
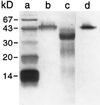
The anionic peroxidase associated with the suberization response in potato (Solanum tuberosum L.) tubers during wound healing has been purified and partially characterized at the biochemical level. It is a 45-kD, class III (plant secretory) peroxidase that is localized to suberizing tissues and shows a preference for feruloyl (o-methoxyphenol)-substituted substrates (order of substrate preference: feruloyl > caffeoyl > p-coumaryl approximately syringyl) such as those that accumulate in tubers during wound healing. There was little influence on oxidation by side chain derivatization, although hydroxycinnamates were preferred over the corresponding hydroxycinnamyl alcohols. The substrate specificity pattern is consistent with the natural substrate incorporation into potato wound suberin. In contrast, the cationic peroxidase(s) induced in response to wound healing in potato tubers is present in both suberizing and nonsuberizing tissues and does not discriminate between hydroxycinnamates and hydroxycinnamyl alcohols. A synthetic polymer prepared using E-[8-(13)C]ferulic acid, H(2)O(2), and the purified anionic enzyme contained a significant amount of cross-linking through C-8, albeit with retention of unsaturation.
Figures



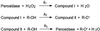
References
-
- Amako K, Chen G-X, Asada K. Separate assays specific for ascorbate peroxidase and guaiacol peroxidase and for the chloroplastic and cytosolic isozymes of ascorbate peroxidase in plants. Plant Cell Physiol. 1994;35:497–504.
-
- Bernards MA, Lewis NG. Alkyl ferulates in wound healing potato tubers. Phytochemistry. 1992;31:3409–3412. - PubMed
-
- Bernards MA, Lewis NG. The macromolecular aromatic domain in suberized tissue: a changing paradigm. Phytochemistry. 1998;47:915–933. - PubMed
-
- Bernards MA, Lopez ML, Zajicek J, Lewis NG. Hydroxycinnamic acid-derived polymers constitute the polyaromatic domain of suberin. J Biol Chem. 1995;270:7382–7386. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

