Oligogalacturonic acid and chitosan reduce stomatal aperture by inducing the evolution of reactive oxygen species from guard cells of tomato and Commelina communis
- PMID: 10482669
- PMCID: PMC59362
- DOI: 10.1104/pp.121.1.147
Oligogalacturonic acid and chitosan reduce stomatal aperture by inducing the evolution of reactive oxygen species from guard cells of tomato and Commelina communis
Abstract
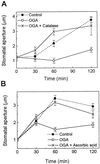
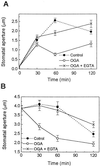
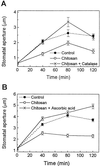
Stomatal opening provides access to inner leaf tissues for many plant pathogens, so narrowing stomatal apertures may be advantageous for plant defense. We investigated how guard cells respond to elicitors that can be generated from cell walls of plants or pathogens during pathogen infection. The effect of oligogalacturonic acid (OGA), a degradation product of the plant cell wall, and chitosan (beta-1,4-linked glucosamine), a component of the fungal cell wall, on stomatal movements were examined in leaf epidermis of tomato (Lycopersicon esculentum L.) and Commelina communis L. These elicitors reduced the size of the stomatal aperture. OGA not only inhibited light-induced stomatal opening, but also accelerated stomatal closing in both species; chitosan inhibited light-induced stomatal opening in tomato epidermis. The effects of OGA and chitosan were suppressed when EGTA, catalase, or ascorbic acid was present in the medium, suggesting that Ca(2+) and H(2)O(2) mediate the elicitor-induced decrease of stomatal apertures. We show that the H(2)O(2) that is involved in this process is produced by guard cells in response to elicitors. Our results suggest that guard cells infected by pathogens may close their stomata via a pathway involving H(2)O(2) production, thus interfering with the continuous invasion of pathogens through the stomatal pores.
Figures


Similar articles
-
Nitric oxide production occurs downstream of reactive oxygen species in guard cells during stomatal closure induced by chitosan in abaxial epidermis of Pisum sativum.Planta. 2009 Mar;229(4):757-65. doi: 10.1007/s00425-008-0855-5. Epub 2008 Dec 16. Planta. 2009. PMID: 19084995
-
Convergence of calcium signaling pathways of pathogenic elicitors and abscisic acid in Arabidopsis guard cells.Plant Physiol. 2002 Dec;130(4):2152-63. doi: 10.1104/pp.012187. Plant Physiol. 2002. PMID: 12481099 Free PMC article.
-
Are grapevine stomata involved in the elicitor-induced protection against downy mildew?Mol Plant Microbe Interact. 2009 Aug;22(8):977-86. doi: 10.1094/MPMI-22-8-0977. Mol Plant Microbe Interact. 2009. PMID: 19589073
-
A sophisticated network of signaling pathways regulates stomatal defenses to bacterial pathogens.Mol Plant. 2015 Apr;8(4):566-81. doi: 10.1016/j.molp.2014.10.012. Epub 2014 Dec 17. Mol Plant. 2015. PMID: 25661059 Review.
-
Mechanism of Stomatal Closure in Plants Exposed to Drought and Cold Stress.Adv Exp Med Biol. 2018;1081:215-232. doi: 10.1007/978-981-13-1244-1_12. Adv Exp Med Biol. 2018. PMID: 30288712 Review.
Cited by
-
Coronatine Facilitates Pseudomonas syringae Infection of Arabidopsis Leaves at Night.Front Plant Sci. 2016 Jun 21;7:880. doi: 10.3389/fpls.2016.00880. eCollection 2016. Front Plant Sci. 2016. PMID: 27446113 Free PMC article.
-
Abscisic Acid biosynthesis and response.Arabidopsis Book. 2002;1:e0058. doi: 10.1199/tab.0058. Epub 2002 Sep 30. Arabidopsis Book. 2002. PMID: 22303212 Free PMC article. No abstract available.
-
Induced resistance in Solanum lycopersicum by algal elicitor extracted from Sargassum fusiforme.ScientificWorldJournal. 2015;2015:870520. doi: 10.1155/2015/870520. Epub 2015 Feb 24. ScientificWorldJournal. 2015. PMID: 25802893 Free PMC article.
-
A comparison of oligogalacturonide- and auxin-induced extracellular alkalinization and growth responses in roots of intact cucumber seedlings.Plant Physiol. 2002 Oct;130(2):895-903. doi: 10.1104/pp.006064. Plant Physiol. 2002. PMID: 12376654 Free PMC article.
-
Cytosolic alkalinization is a common and early messenger preceding the production of ROS and NO during stomatal closure by variable signals, including abscisic acid, methyl jasmonate and chitosan.Plant Signal Behav. 2009 Jun;4(6):561-4. doi: 10.4161/psb.4.6.8847. Epub 2009 Jun 22. Plant Signal Behav. 2009. PMID: 19816133 Free PMC article.
References
-
- Adam A, Farkas T, Somlyai G, Hevesi M, Kiraly Z. Consequence of O2− generation during a bacterially induced hypersensitive reaction in tobacco: deterioration of membrane lipids. Physiol Mol Pathol. 1989;34:13–26.
-
- Agrios GN (1997) Plant Pathology, Ed 4. Academic Press, San Diego, pp 46–52
-
- Assmann SM. Signal transduction in guard cells. Annu Rev Cell Biol. 1993;9:345–375. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

