Spider mite-induced (3S)-(E)-nerolidol synthase activity in cucumber and lima bean. The first dedicated step in acyclic C11-homoterpene biosynthesis
- PMID: 10482672
- PMCID: PMC59365
- DOI: 10.1104/pp.121.1.173
Spider mite-induced (3S)-(E)-nerolidol synthase activity in cucumber and lima bean. The first dedicated step in acyclic C11-homoterpene biosynthesis
Abstract
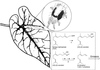
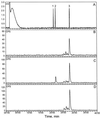
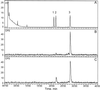
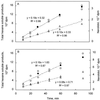
Many plant species respond to herbivory with de novo production of a mixture of volatiles that attracts carnivorous enemies of the herbivores. One of the major components in the blend of volatiles produced by many different plant species in response to herbivory by insects and spider mites is the homoterpene 4,8-dimethyl-1,3(E), 7-nonatriene. One study (J. Donath, W. Boland [1995] Phytochemistry 39: 785-790) demonstrated that a number of plant species can convert the acyclic sesquiterpene alcohol (3S)-(E)-nerolidol to this homoterpene. Cucumber (Cucumis sativus L.) and lima bean (Phaseolus lunatus L.) both produce 4,8-dimethyl-1,3(E),7-nonatriene in response to herbivory. We report the presence in cucumber and lima bean of a sesquiterpene synthase catalyzing the formation of (3S)-(E)-nerolidol from farnesyl diphosphate. The enzyme is inactive in uninfested cucumber leaves, slightly active in uninfested lima bean leaves, and strongly induced by feeding of the two-spotted spider mite (Tetranychus urticae Koch) on both plant species, but not by mechanical wounding. The activities of the (3S)-(E)-nerolidol synthase correlated well with the levels of release of 4, 8-dimethyl-1,3(E),7-nonatriene from the leaves of the different treatments. Thus, (3S)-(E)-nerolidol synthase is a good candidate for a regulatory role in the release of the important signaling molecule 4,8-dimethyl-1,3(E),7-nonatriene.
Figures


References
-
- Alborn T, Turlings TCJ, Jones TH, Stenhagen G, Loughrin JH, Tumlinson JH. An elicitor of plant volatiles from beet armyworm oral secretion. Science. 1997;276:945–949.
-
- Bauer K, Garbe D, Surburg H (1990) Common Fragrance and Flavor Materials. Preparations, Properties and Uses, Ed 2. VCH Verlaggesellschaft, Weinheim, Germany
-
- Bouwmeester HJ, Wallaart TE, Janssen MHA, Van Loo B, Jansen BJM, Posthumus MA, Schmidt CO, De Kraker J-W, König WA, Franssen MCR (1999) Amorpha-4,11-diene synthases catalyses the first probable step in artemisinin biosynthesis. Phytochemistry (in press) - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

