Solubilization and partial characterization of homogalacturonan-methyltransferase from microsomal membranes of suspension-cultured tobacco cells
- PMID: 10482684
- PMCID: PMC59378
- DOI: 10.1104/pp.121.1.281
Solubilization and partial characterization of homogalacturonan-methyltransferase from microsomal membranes of suspension-cultured tobacco cells
Abstract
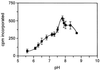
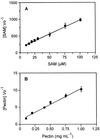
The transfer of a methyl group from S-adenosyl-L-methionine onto the carboxyl group of alpha-1,4-linked-galactosyluronic acid residues in the pectic polysaccharide homogalacturonan (HGA) is catalyzed by an enzyme commonly referred to as pectin methyltransferase. A pectin methyltransferase from microsomal membranes of tobacco (Nicotiana tabacum) was previously characterized (F. Goubet, L.N. Council, D. Mohnen [1998] Plant Physiol 116: 337-347) and named HGA methyltransferase (HGA-MT). We report the solubilization of HGA-MT from tobacco membranes. Approximately 22% of the HGA-MT activity in total membranes was solubilized by 0.65% (w/v) 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid containing 1 mM dithioerythritol. The addition of phosphatidylcholine and the methyl acceptors HGA or pectin (30% degree of esterification) to solubilized enzyme increased HGA-MT activity to 35% of total membrane-bound HGA-MT activity. Solubilized HGA-MT has a pH optimum of 7.8, an apparent K(m) for S-adenosyl-L-methionine of 18 microM, and an apparent V(max) of 0. 121 pkat mg(-1) of protein. The apparent K(m) for HGA and for pectin is 0.1 to 0.2 mg mL(-1). Methylated product was solubilized with boiling water and ammonium oxalate, two conditions used to solubilize pectin from the cell wall. The release of 75% to 90% of the radioactivity from the product pellet by mild base treatment showed that the methyl group was incorporated as a methyl ester rather than a methyl ether. The fragmentation of at least 55% to 70% of the radiolabeled product by endopolygalacturonase, and the loss of radioactivity from the product by treatment with pectin methylesterase, demonstrated that the bulk of the methylated product produced by the solubilized enzyme was pectin.
Figures



Similar articles
-
Subcellular localization and topology of homogalacturonan methyltransferase in suspension-cultured Nicotiana tabacum cells.Planta. 1999 Jul;209(1):112-7. doi: 10.1007/s004250050612. Planta. 1999. PMID: 10467037
-
Characterization of pectin methyltransferase from soybean hypocotyls.Planta. 2000 Apr;210(5):782-91. doi: 10.1007/s004250050680. Planta. 2000. PMID: 10805450
-
The Homogalacturonan Deconstruction System of Paenibacillus amylolyticus 27C64 Requires No Extracellular Pectin Methylesterase and Has Significant Industrial Potential.Appl Environ Microbiol. 2020 Jun 2;86(12):e02275-19. doi: 10.1128/AEM.02275-19. Print 2020 Jun 2. Appl Environ Microbiol. 2020. PMID: 32303547 Free PMC article.
-
The Multifaceted Role of Pectin Methylesterase Inhibitors (PMEIs).Int J Mol Sci. 2018 Sep 21;19(10):2878. doi: 10.3390/ijms19102878. Int J Mol Sci. 2018. PMID: 30248977 Free PMC article. Review.
-
Homogalacturonan-modifying enzymes: structure, expression, and roles in plants.J Exp Bot. 2014 Oct;65(18):5125-60. doi: 10.1093/jxb/eru272. Epub 2014 Jul 23. J Exp Bot. 2014. PMID: 25056773 Free PMC article. Review.
Cited by
-
Rice putative methyltransferase gene OsTSD2 is required for root development involving pectin modification.J Exp Bot. 2016 Oct;67(18):5349-5362. doi: 10.1093/jxb/erw297. Epub 2016 Aug 6. J Exp Bot. 2016. PMID: 27497286 Free PMC article.
-
Microarray analysis of developing flax hypocotyls identifies novel transcripts correlated with specific stages of phloem fibre differentiation.Ann Bot. 2008 Sep;102(3):317-30. doi: 10.1093/aob/mcn110. Epub 2008 Jun 30. Ann Bot. 2008. PMID: 18593690 Free PMC article.
-
Amplification, sequencing and characterization of pectin methyl esterase inhibitor 51 gene in Tectona grandis L.f.Saudi J Biol Sci. 2021 Oct;28(10):5451-5460. doi: 10.1016/j.sjbs.2021.07.015. Epub 2021 Jul 10. Saudi J Biol Sci. 2021. PMID: 34588855 Free PMC article.
-
Pectin: cell biology and prospects for functional analysis.Plant Mol Biol. 2001 Sep;47(1-2):9-27. Plant Mol Biol. 2001. PMID: 11554482 Review.
-
QUASIMODO 3 (QUA3) is a putative homogalacturonan methyltransferase regulating cell wall biosynthesis in Arabidopsis suspension-cultured cells.J Exp Bot. 2011 Oct;62(14):5063-78. doi: 10.1093/jxb/err211. Epub 2011 Jul 1. J Exp Bot. 2011. PMID: 21725030 Free PMC article.
References
-
- An J, Zhang L, O'Neill MA, Albersheim P, Darvill AG. Isolation and structural characterization of endo-rhamnogalacturonase-generated fragments of the backbone of rhamnogalacturonan I. Carbohydr Res. 1994;264:83–96. - PubMed
-
- BeMiller JN (1986) An Introduction to Pectins: Structure and Properties. In ML Fishman, JJ Jen, eds, Chemistry and Function of Pectins. American Chemical Society, Washington, DC, pp 2–12
-
- Blumenkrantz N, Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973;54:484–489. - PubMed
-
- Bolwell GP, Dalessandro G, Northcote DH. Decrease of polygalacturonic acid synthase during xylem differentiation in sycamore. Phytochemistry. 1985;24:699–702.
-
- Bourlard T, Schaumann-Gaudinet A, Bruyant-Vannier M-P, Morvan C. Various pectin methyltransferase activities with affinity for low and highly methylated pectins. Plant Cell Physiol. 1997;38:259–267.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

